Permethrin Therapeutic Cheat Sheet
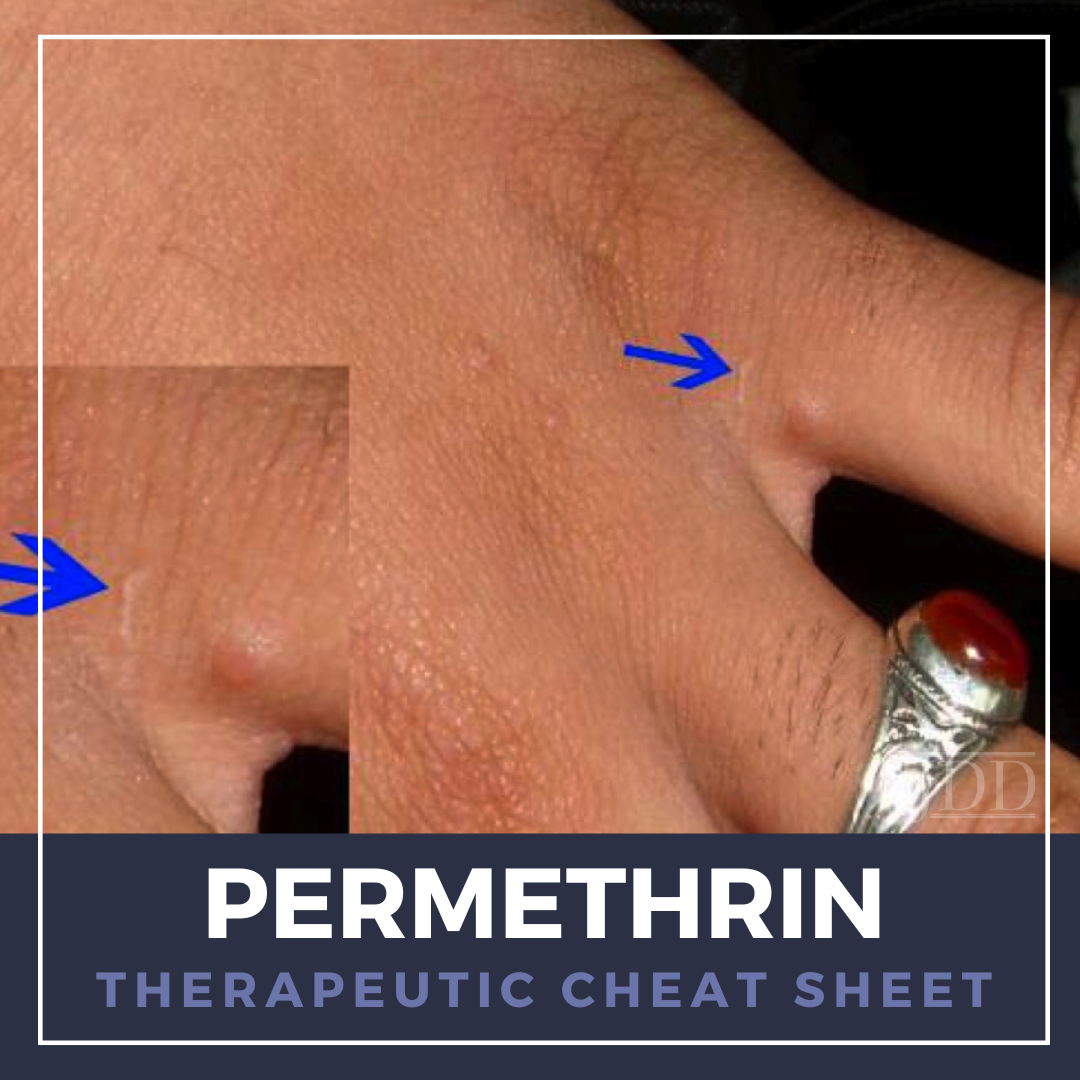 Permethrin is a synthetic neurotoxic pyrethroid derived from chrysanthemums with significant dermatologic value for its powerful insecticide properties when used topically. We continue our series, Therapeutic Cheat Sheet, with a closer look at permethrin, which is FDA-approved for the treatment of scabies and pediculosis capitis infestations.
Permethrin Therapeutic Cheat Sheet
Compiled by: Dillo …
Permethrin is a synthetic neurotoxic pyrethroid derived from chrysanthemums with significant dermatologic value for its powerful insecticide properties when used topically. We continue our series, Therapeutic Cheat Sheet, with a closer look at permethrin, which is FDA-approved for the treatment of scabies and pediculosis capitis infestations.
Permethrin Therapeutic Cheat Sheet
Compiled by: Dillo …
 Permethrin is a synthetic neurotoxic pyrethroid derived from chrysanthemums with significant dermatologic value for its powerful insecticide properties when used topically. We continue our series, Therapeutic Cheat Sheet, with a closer look at permethrin, which is FDA-approved for the treatment of scabies and pediculosis capitis infestations.
Permethrin Therapeutic Cheat Sheet
Compiled by: Dillo …
Permethrin is a synthetic neurotoxic pyrethroid derived from chrysanthemums with significant dermatologic value for its powerful insecticide properties when used topically. We continue our series, Therapeutic Cheat Sheet, with a closer look at permethrin, which is FDA-approved for the treatment of scabies and pediculosis capitis infestations.
Permethrin Therapeutic Cheat Sheet
Compiled by: Dillo … 

 Ketoconazole is an imidazole antifungal that was initially FDA-approved in an oral formulation for the treatment of systemic fungal infections. While oral ketoconazole has largely fallen out of use due to safety concerns, including hepatotoxicity, endocrine dysregulation, and drug interactions, topical ketoconazole has proven to be a safe and effective treatment for superficial fungal infections. …
Ketoconazole is an imidazole antifungal that was initially FDA-approved in an oral formulation for the treatment of systemic fungal infections. While oral ketoconazole has largely fallen out of use due to safety concerns, including hepatotoxicity, endocrine dysregulation, and drug interactions, topical ketoconazole has proven to be a safe and effective treatment for superficial fungal infections. … 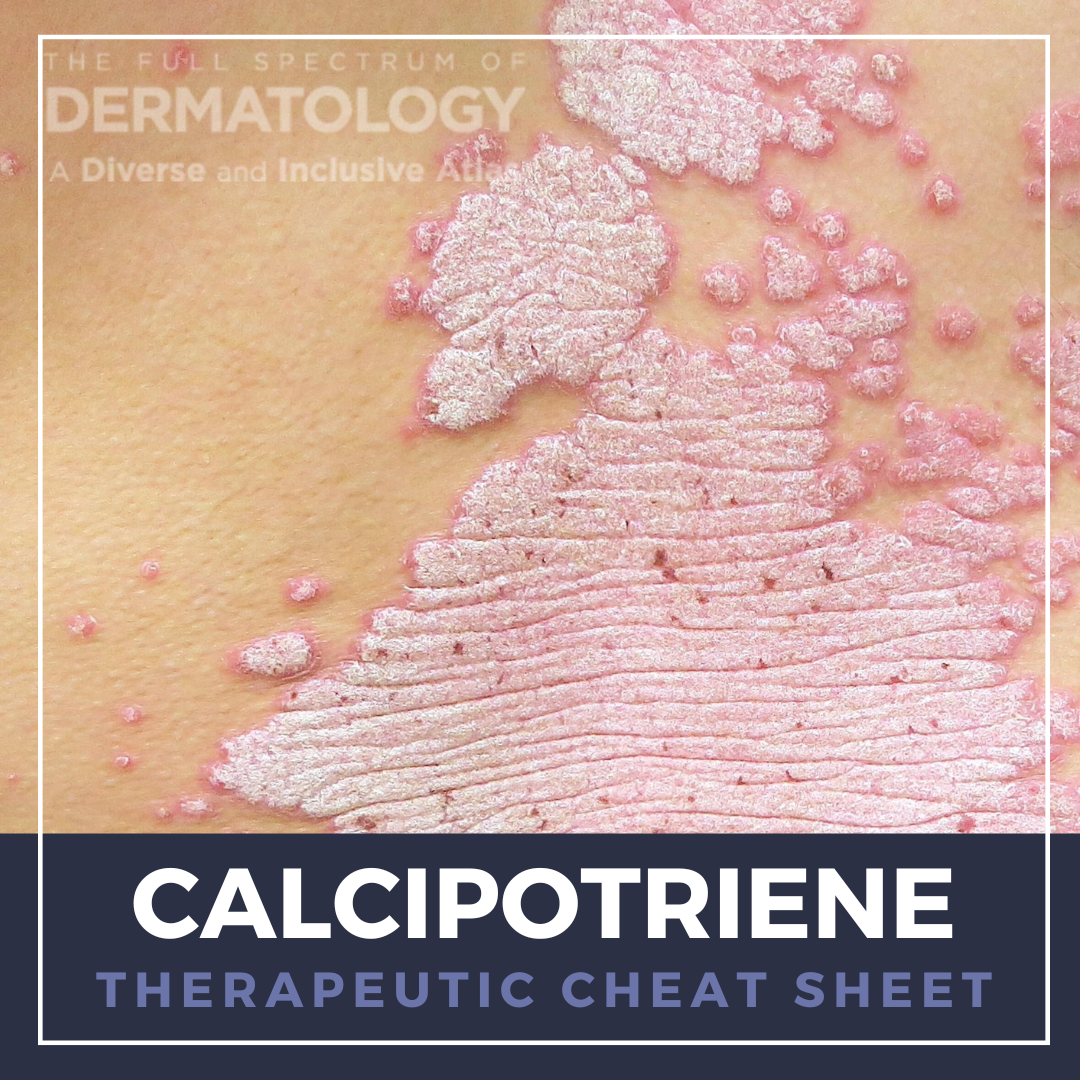 Calcipotriene is a synthetic Vitamin D3 analogue with various functions, including promoting cellular differentiation and reducing inflammatory cytokines. The unique mechanism of action of calcipotriene has expanded its utility beyond psoriasis to off-label uses for other dermatologic conditions. Calcipotriene is well tolerated and can be used in conjunction with other treatment modalities. We con …
Calcipotriene is a synthetic Vitamin D3 analogue with various functions, including promoting cellular differentiation and reducing inflammatory cytokines. The unique mechanism of action of calcipotriene has expanded its utility beyond psoriasis to off-label uses for other dermatologic conditions. Calcipotriene is well tolerated and can be used in conjunction with other treatment modalities. We con … 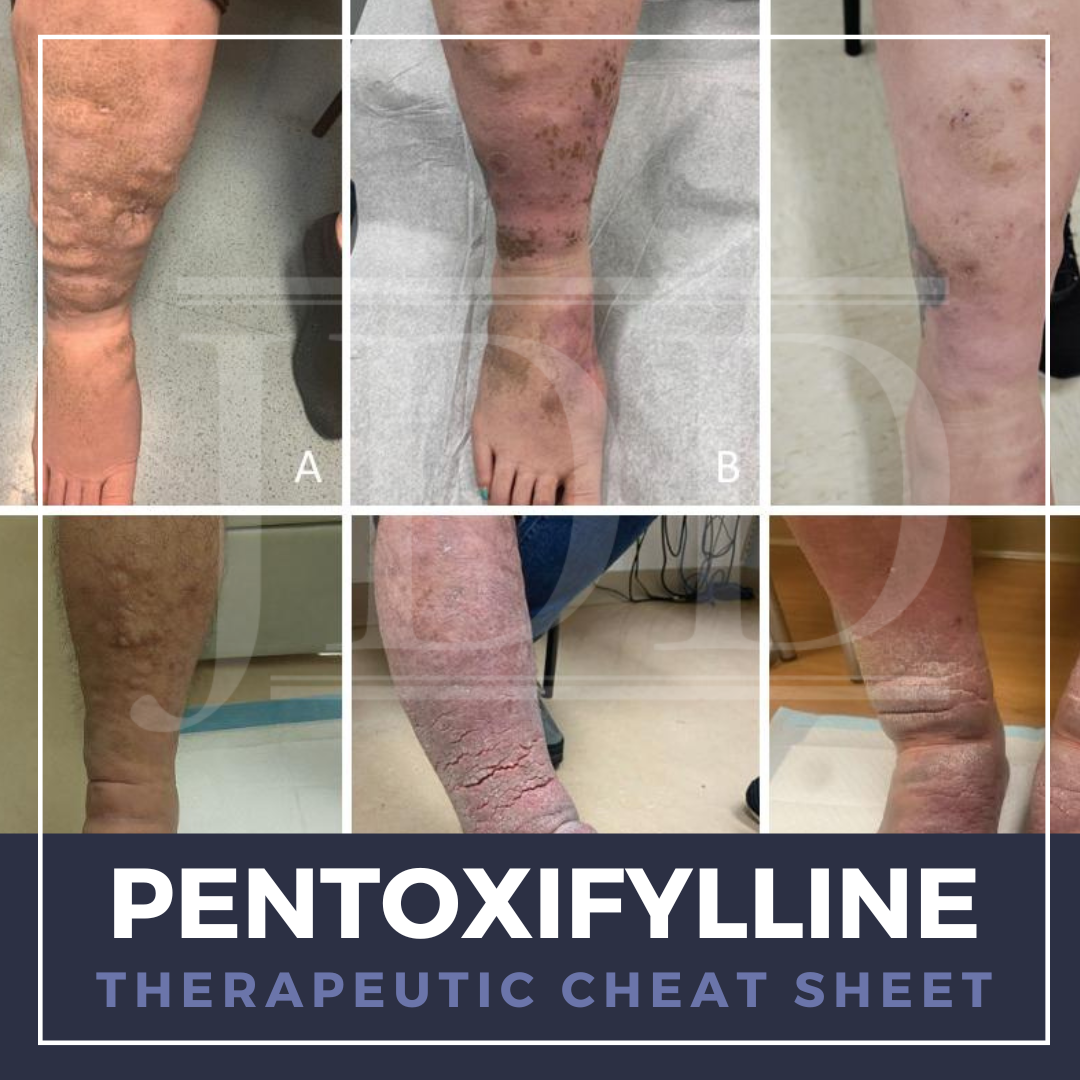 Pentoxifylline is a methyl-xanthine derivative traditionally used to treat symptoms of peripheral vascular disease and has emerged as a valuable therapeutic agent in dermatology for its anti-inflammatory and immunomodulatory properties. We continue our series, Therapeutic Cheat Sheet, with a closer look at pentoxifylline, which is FDA-approved for the treatment of intermittent claudication and is …
Pentoxifylline is a methyl-xanthine derivative traditionally used to treat symptoms of peripheral vascular disease and has emerged as a valuable therapeutic agent in dermatology for its anti-inflammatory and immunomodulatory properties. We continue our series, Therapeutic Cheat Sheet, with a closer look at pentoxifylline, which is FDA-approved for the treatment of intermittent claudication and is …  Alopecia areata (AA) is a nonscarring alopecia driven by autoreactive cytotoxic CD8 T cells against hair follicle antigens. With an unpredictable clinical course, which can include alopecia totalis and universalis, AA can be debilitating to patients’ quality of life. Historically, glucocorticosteroids, cyclosporine, methotrexate, and azathioprine were used off-label for non-targeted immunosuppre …
Alopecia areata (AA) is a nonscarring alopecia driven by autoreactive cytotoxic CD8 T cells against hair follicle antigens. With an unpredictable clinical course, which can include alopecia totalis and universalis, AA can be debilitating to patients’ quality of life. Historically, glucocorticosteroids, cyclosporine, methotrexate, and azathioprine were used off-label for non-targeted immunosuppre …