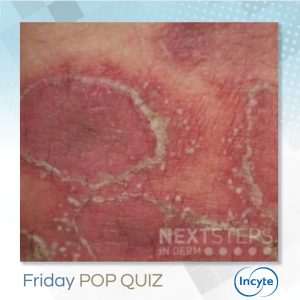
The correct answer is C. IL-36.
IL-36. Spesolimab is a novel, selective humanized anti–interleukin-36 receptor monoclonal antibody recently approved by the for the treatment of adults with GPP.1
IL-17 inhibitors approved for plaque psoriasis include secukinumab, ixekizumab, and brodalumab. Bimekizumab is also an IL-17 inhibitor in ongoing clinical trials for plaque psoriasis. IL-23 inhibitors approved for plaque psoriasis include guselkumab, risankizumab, ustekinumab, and tildrakizumab.2 None of these are specifically approved for plaque psoriasis. Tralokinumab is an IL-13 blocker FDA approved for atopic dermatitis. Lebrikizumab is another fully humanized anti-IL-13 antibody that does not block the binding of the cytokine to the receptor but instead impairs the heterodimerization of IL-4Rα and IL-13Rα1, thereby inhibiting signal transduction, but is still in clinical trials.3 Nemolizumab is not currently FDA approved in the United States but is a subcutaneously administered humanized anti-interleukin-31 (IL-31) receptor A (IL-31RA) monoclonal antibody that is being developed for the treatment of skin diseases, including atopic dermatitis (AD), AD associated pruritus, prurigo nodularis, chronic kidney disease associated pruritus, and systemic sclerosis.4
Brought to you by our brand partner

