The correct answer is A. Ruxolitinib
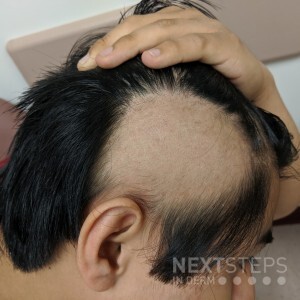
The photo depicts a patient with Alopecia Areata. The FDA approved Baricitinib, a JAK 1/2 inhibitor, in June 2022 for the treatment of alopecia areata. Baricitinib blocks JAK 1/2, just as Ruxolitinib, which was FDA approved for the treatment of atopic dermatitis in September 2021.
Abrocitinib, while a JAK inhibitor, only inhibits JAK1 as opposed to JAK 1/2.
Apremilast is an inhibitor of phosphodiesterase 4 (PDE4), not JAK.
Adalimumab is an inhibitor of TNFalpha, not JAK.
Tofacitinib is a JAK inhibitor, but inhibits JAK1/3, as opposed to JAK 1/2.
References:
King B, Ohyama M, Kwon O, Zlotogorski A, Ko J, Mesinkovska NA, Hordinsky M, Dutronc Y, Wu WS, McCollam J, Chiasserini C, Yu G, Stanley S, Holzwarth K, DeLozier AM, Sinclair R; BRAVE-AA Investigators. Two Phase 3 Trials of Baricitinib for Alopecia Areata. N Engl J Med. 2022 May 5;386(18):1687-1699. doi: 10.1056/NEJMoa2110343. Epub 2022 Mar 26. PMID: 35334197.
