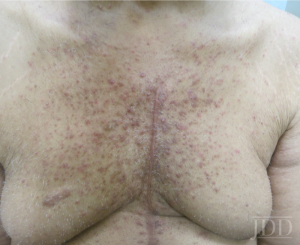
The correct answer is D: Bevacizumab would not cause this skin eruption.
This patient has a papulopustular drug eruption associated with erlotinib, an epidermal growth factor receptor inhibitor (EGFR-I). Acneiform eruptions with EGFR-I use can be seen in more than 75% of patients treated with these agents and is more common with the monoclonal antibodies, cetuximab and panitumumab, than the low molecular weight tyrosine kinase receptor inhibitors like erlotinib and gefitinib. EGFR is involved in regulating keratinocyte proliferation and normal differentiation of the hair follicle. Inhibition of EGFR allows for the production of several inflammatory cytokines and recruitment of inflammatory cells in the dermis coupled with disruption of hair follicle growth which leads to the characteristic papulopustular eruption localized mostly to hair follicles.
The eruption, which mostly affects the trunk, face and scalp accompanied by pruritus, burning or pain, usually occurs within the first two weeks of treatment and resolves within a month once EGFR-I therapy is stopped. Discrete erythematous papules and pustules in areas with high numbers of sebaceous glands are seen on exam without comedones. Patients can experience mild, almost prodromal symptoms of burning or erythema before the appearance of papules and pustules. Some risk factors for development include higher medication doses and sun exposure.
Other cutaneous side effects of EGFR-I therapy includes both scarring and non-scarring alopecias, hair textural changes, paronychia, pyogenic granuloma-like lesions, pruritus, trichomegaly, seborrheic dermatitis, photosensitivity and stomatitis.
There are four grades used to classify the severity of these reactions. Grade 1 involves papules and pustules covering less than 10% BSA, while grade 2 involves papules and pustules over 10-30% BSA but also with symptoms of pain or pruritus that limits daily activities. Grade 3 affects more than 30% BSA with symptoms of pain and pruritus, difficulty with self-care and local superinfection warranting oral antibiotics. Grade 4 reactions include greater than 30% BSA affected with pain and pruritus plus extensive superinfection requiring IV antibiotics. Interestingly, for NSLC and metastatic colon cancer, severity of the papulopustular rash has been correlated with better antitumor responses.
Of note, development of this eruption is not a contraindication to continue EGFR-I chemotherapy nor does it preclude future use of a different EGFR-I. However, it is important to mitigate symptoms or even prevent development since this eruption can lead to temporary discontinuation of the medication and patient reported lower quality of life. One study looking at EFGR-I therapy dermatologic toxicities found that rash as opposed to xerosis, paronychia and pruritus, led to a greater decrease in quality of life scores and that emotional well being and daily functioning in activities was affected by the papulopustular eruption.
Since there is such a high incidence and frequent patient discomfort associated with this eruption, it often warrants treatment based on severity. For mild grade 1 reactions, topical acne medications like clindamycin, benzoyl peroxide, metronidazole and erythromycin can be used. In grade 2 reactions, the same topical medications should be used in addition to an oral tetracycline like doxycycline or minocycline and an antihistamine for pruritus. Additionally, topical steroids and/or retinoids in conjunction with an oral tetracycline has been effective. Reports have also showed that oral isotretinoin is efficacious however since EGFR-I therapy can also cause paronychia and xerosis, patients should be appropriately counseled. For grade 3 reactions, EGFR-I treatment may need to be delayed to allow for treatment with oral tetracyclines to reduce inflammation. In grade 4 reactions, treatment with EGFR-Is should be discontinued and patient may require management by a burn unit.
Answer choice D, bevacizumab, is a humanized monoclonal antibody against vascular endothelial growth factor (VEGF). Bevacizumab is FDA- approved used to treat metastatic colon cancer, lung cancer, renal cell carcinoma, certain brain cancers and age-related macular degeneration. Bevacizumab is not known for causing cutaneous reactions but several potential side effects include hemorrhage, gastrointestinal perforations, impaired wound healing, hypertension, blood clots, thrombocytopenia, diarrhea and xerosis.
References:
- Balagula Y, et al. Clinical presentation and management of dermatological toxicities of epidermal growth factor receptor inhibitors. Int J Dermatol. 2011;50(2):129-46.
- Hu J, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56(2):317-26.
- Bolognia J et al. (2012). Dermatology. Philadelphia: Elsevier Saunders.
- Joshi S, et al. Effects of epidermal growth factor receptor inhibitor-induced dermatologic toxicities on quality of life. Cancer. 2010;116(16):3916-23.
