Alopecia areata (AA) is a common autoimmune disorder. Although its pathogenesis is not fully understood, AA involves CD8 T cell-mediated destruction of the hair follicle. Several treatment options exist; however, there is minimal evidence in the pediatric population. Currently, there are no curative treatments for AA. The literature suggests that Janus kinase (JAK) inhibitors may be an effective treat-ment for AA, but evidence in pediatric patients is limited. Here, JDD authors Yazmeen Tembunde BS and Chesahna Kindred MD MBA FAAD report a case of severe pediatric AA treated with topical ruxolitinib, a JAK inhibitor.
The patient was a 5-year-old female with a past medical history of atopic dermatitis and alopecia areata diagnosed at age 3 years by her pediatrician. Laboratory evaluation was within normal limits, including complete blood count, comprehensive metabolic panel, antibody screen, thyroid profile, and vitamin D levels. The patient was started on clobetasol 0.05% topical cream, which was applied to the areas of hair loss 1-2 times daily. A year later, the patient presented to us with patchy loss of scalp hair. Her mother reported that the AA persistently deteriorated despite treatment adherence. The physical exam was notable for coalescing oval patches of non-scarring hair loss involving about a third of her scalp hair. We initiated ruxolitinib 1.5% topical cream applied to the affected areas twice daily for 30 days, fluocinonide 0.05% topical solution applied to the affected areas once daily for 30 days, and oral fexofenadine 30 mg twice a day. The patient tolerated treatment well and without adverse effects.
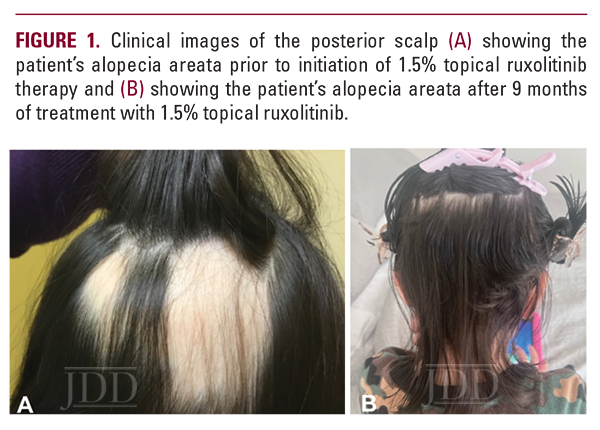
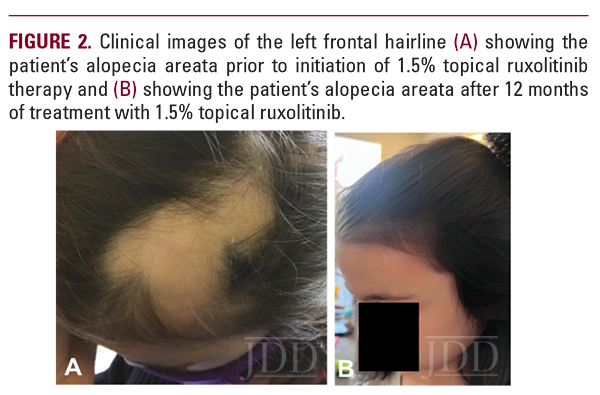
At her 3-month follow-up appointment, all alopecic lesions displayed diffuse new growth, except one new lesion on the occipital scalp and one persistent lesion on the left frontal hairline. At 6 months, the patient discontinued fluocinonide and fexofenadine and continued ruxolitinib to the affected areas. She had one persistent lesion on the left frontal hairline that was also noted at 9 months. The patient continued to have noticeable improvements with no side effects (Fig 1B). At 12 months, the persistent frontal hairline lesion resolved (Figure 2b). At this time, the patient discontinued ruxolitinib treatment. A few weeks later, however, new lesions appeared on the occipital scalp.
INTRODUCTION
Alopecia areata (AA) is an autoimmune, nonscarring hair loss condition that is more prevalent in children compared to adults.1,2 It has an unpredictable clinical course with limited safe and effective treatment options. With the significant psychosocial consequences pediatric AA patients may face, safe and effective therapies are needed to prevent further morbidity.1 The literature suggests that Janus kinase (JAK) inhibitors may be an effective treatment for AA, but evidence in pediatric patients is limited.1 Topical JAK inhibitor formulations, which are FDA-approved for atopic dermatitis, may have fewer risks and may be more tolerable for pediatric patients compared to oral formulations.1,3,4 However, there is a paucity of case reports regarding the use of topical JAK inhibitors in pediatric patients with AA. We report a pediatric patient with severe AA who was successfully treated with ruxolitinib 1.5% topical cream, a JAK inhibitor.
CASE REPORT
The patient was a 5-year-old female with a past medical history of atopic dermatitis and alopecia areata diagnosed at age 3 years by her pediatrician. Laboratory evaluation was within normal limits, including complete blood count, comprehensive metabolic panel, antibody screen, thyroid profile, and vitamin D levels. The patient was started on clobetasol 0.05% topical cream, which was applied to the areas of hair loss 1-2 times daily. A year later, the patient presented to us with patchy loss of scalp hair. Her mother reported that the AA persistently deteriorated despite treatment adherence. The physical exam was notable for coalescing oval patches of non-scarring hair loss involving about a third of her scalp hair. We initiated ruxolitinib 1.5% topical cream applied to the affected areas twice daily for 30 days, fluocinonide 0.05% topical solution applied to the affected areas once daily for 30 days, and oral fexofenadine 30 mg twice a day. The patient tolerated treatment well and without adverse effects.
At her 3-month follow-up appointment, all alopecic lesions displayed diffuse new growth, except one new lesion on the occipital scalp and one persistent lesion on the left frontal hairline. At 6 months, the patient discontinued fluocinonide and fexofenadine and continued ruxolitinib to the affected areas. She had one persistent lesion on the left frontal hairline that was also noted at 9 months. The patient continued to have noticeable improvements with no side effects (Fig 1B). At 12 months, the persistent frontal hairline lesion resolved (Figure 2b). At this time, the patient discontinued ruxolitinib treatment. A few weeks later, however, new lesions appeared on the occipital scalp.
 DISCUSSION
DISCUSSION
The patient was initially treated with combination treatment followed by ruxolitinib 1.5% topical cream as monotherapy. Her initial lesions and new lesions responded to ruxolitinib as monotherapy, suggesting that topical ruxolitinib may be an efficacious treatment option for pediatric AA. This topical JAK inhibitor therapy seems to avoid the adverse effects associated with oral JAK inhibitor therapies and is generally well tolerated by children, compared to the poor tolerance seen with irritating topical therapies or painful procedures.4,5 Thus, topical ruxolitinib may be a promising treatment option for pediatric patients with AA. AA has an unpredictable clinical course and can resolve spontaneously, thus, a true response to treatment can be difficult to ascertain.2 Further evaluation in a controlled study with more patients is warranted to fully determine the potential efficacy of this treatment.
DISCLOSURES
Dr Chesahna Kindred has served on the advisory board for Lilly, UCB, Sun Pharmaceuticals, Abbvie, Pfizer, and Janssen; on the speaker’s bureau for Lilly, Nutrafol, Aerolase, and Sun Pharmaceuticals.
REFERENCES
-
- Barton VR, Toussi A, Awasthi S, Kiuru M. Treatment of pediatric alopecia areata: A systematic review. J Am Acad Dermatol. 2022;86(6):1318-1334. doi:10.1016/j. jaad.2021.04.077
- Spano F, Donovan JC. Alopecia areata: Part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61(9):751-755.
- Bayart CB, DeNiro KL, Brichta L, Craiglow BG, Sidbury R. Topical Janus kinase inhibitors for the treatment of pediatric alopecia areata. J Am Acad Dermatol. 2017;77(1):167-170. doi:10.1016/j.jaad.2017.03.024
- Putterman E, Castelo-Soccio L. Topical 2% tofacitinib for children with alopecia areata, alopecia totalis, and alopecia universalis. J Am Acad Dermatol. 2018;78(6):1207-1209. e1. doi:10.1016/j.jaad.2018.02.031
- Hamilton CE, Craiglow BG. JAK Inhibitors for the treatment of pediatric alopecia areata. Journal of Investigative Dermatology Symposium Proceedings. 2020;20(1):S31-S36. doi:10.1016/j.jisp.2020.04.005
SOURCE
Tembunde, Y., & Kindred, C. (2024). Ruxolitinib 1.5% Topical Cream for the Treatment of Pediatric Alopecia Areata. Journal of Drugs in Dermatology: JDD, 23(5), 378-379.
Content and images published with permission from the Journal of Drugs in Dermatology.
Adapted from original article for length and style.
Did you enjoy this JDD Case Report? You can find more
here.
 DISCUSSION
DISCUSSION

