Seborrheic dermatitis, a common but often overlooked condition, has received little attention in recent decades despite its significant impact on patients, including itch and cosmetic concerns. At the 2024 Skin of Color Update, Dr. Shawn Kwatra, Professor and Chair of Dermatology at the University of Maryland School of Medicine, provided a fresh perspective on its evaluation and management in patients with skin of color.
Seborrheic Dermatitis Statistics and Associations
Seborrheic dermatitis affects approximately 3-5%1 of adults and 60-70% of infants2. Seborrheic dermatitis is linked to neurological disorders like Parkinson’s disease and often improves as these conditions are treated. Additionally, research indicates that neurons may be involved in triggering cutaneous inflammation in seborrheic dermatitis.
Male sex, HIV positivity, and environmental influences including diet are associated with a seborrheic dermatitis. Research into the gut microbiome of patients with this condition has revealed an increase of Candida albicans, which typically coexists with Malassezia species3.
Seborrheic Dermatitis Presentation
Dr. Kwatra emphasized the varied diagnostic terms for seborrheic dermatitis, including sebopsoriasis, psoriasis, and seborrheic dermatitis itself. Patients may present with isolated flaking, erythema, or features resembling eczema or psoriasis, underscoring the condition’s heterogeneity. In patients with more scaling than itch the disease is likely driven by Type 17 inflammation, while patients with more itch than scaling often have Type 2 inflammation. The differential diagnosis of seborrheic dermatitis often includes other conditions which present with hypopigmentation including pityriasis alba.
Seborrheic Dermatitis in Patients with Skin of Color
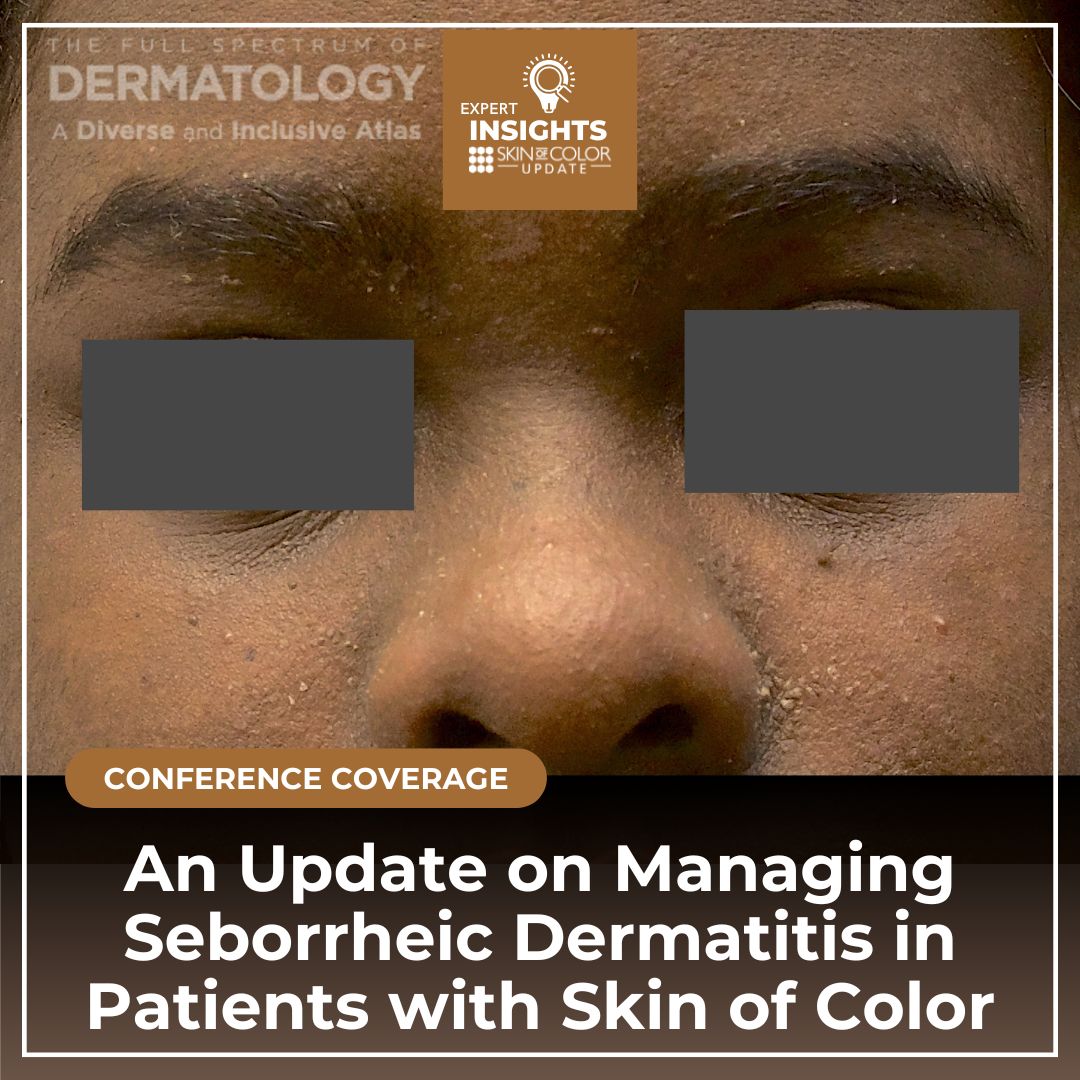
Seborrheic dermatitis is among the top five skin conditions affecting patients with skin of color. While erythema is less detectable in skin of color, hypopigmentation is more common and warrants caution when using topical corticosteroids.
Seborrheic Dermatitis Pathogenesis
In seborrheic dermatitis, the fatty bilayer is dysregulated, with disrupted sebum triglycerides and altered fatty acid hydrolysis leading to keratinocyte proliferation and inflammation. The key driver of inflammation in seborrheic dermatitis is increased Malassezia spp, alongside decreased ceramides and elevated pro-inflammatory cytokines, including IL-17, IL-13, IL-2 and IFN- γ. These pathways offer potential therapeutic targets for managing the condition.
Seborrheic Dermatitis Goals of Treatment
Seborrheic dermatitis can impact social lives, personal relationships, clothing choices, hygiene routine and increase perceived social stigma. Treatment goals include modulating sebum production, controlling inflammation, addressing barrier dysfunction, reducing Malassezia on the skin, and alleviating itch and scaling.
Pre-2024 Standard of Care Treatments for Seborrheic Dermatitis
Dr. Kwatra discussed the various treatments for seborrheic dermatitis including over-the-counter shampoos and emollients, topical and systemic antifungals, corticosteroids and more recently, off-label use of calcineurin inhibitors, PDE-4 inhibitors and systemic retinoids.4,5 Highlights are found below:
-
- A phase 2 study comparing hydrocortisone 1% ointment to tacrolimus 0.1% ointment showed similar efficacy, with hydrocortisone performing slightly better.6
-
- Another study involving 114 adults with severe facial seborrheic dermatitis compared tacrolimus 0.1% ointment and ciclopiroxamine 1% cream.7 After a one-week course of desonide 0.05% cream, patients were randomized to either tacrolimus or ciclopiroxamine. Patients in the tacrolimus group had fewer relapses, showing tacrolimus to be more effective as maintenance therapy for facial seborrheic dermatitis. Overall, this suggests targeting the inflammatory axis first in the treatment of seborrheic dermatitis may achieve better treatment results.
-
- A study showed that the phosphodiesterase inhibitor crisaborole had a higher clearance rate compared to existing treatments, along with improved hyperpigmentation and reduced itch.8
-
- Oral anti-fungal can also be used in the treatment of seborrheic dermatitis. In a study evaluating itraconazole, 200mg/day was combined with topical hydrocortisone 1% and ketoconazole 2% cream for up to 3 months.9 Significant improvement was seen in the oral itraconazole group compared to the placebo group, which received only hydrocortisone 1% ointment and ketoconazole 2% cream. Oral itraconazole likely modulates the gut microbiome, specifically targeting Candida spp.
-
- A study comparing a single 300mg oral dose of fluconazole weekly for two weeks to placebo showed no significant difference between the groups.10 (PMID 17645378). In clinical practice, Dr Kwatra recommends fluconazole 200mg once weekly for patients under 200 pounds, and 300mg once weekly for those over 200 pounds.
Next Steps After Treatment
Dr. Kwatra recommended Roflumilast 0.3% foam, FDA-approved for seborrheic dermatitis for patients who not responded to topical antifungals and corticosteroids. A study showed that once-daily Roflumilast was superior to vehicle in achieving an IGA of “Clear” or “Almost clear” from baseline at 8 weeks in patients with seborrheic dermatitis11. Roflumilast vehicle does not contain propylene glycol and is better tolerated than other phosphodiesterase 4 inhibitors, with minimal side effects. No significant adverse events including burning and stinging were reported in the clinical trial. Roflumilast has also been shown to improve both hypo- and hyperpigmentation associated with seborrheic dermatitis in patients of color and can serve as an alternative to tacrolimus for managing hypopigmentation.
Future Treatment: JAK and PDE4 Inhibitors
-
- Ruxolitinib 1.5% cream, a JAK1 and JAK2 inhibitor, is currently in phase 2 trials
- PF-07038124 0.02% ointment, a PDE4 inhibitor, is also in phase 2 trials
In conclusion, Dr. Kwatra emphasized that while patients may be bothered by seborrheic dermatitis, they often do not bring it up to their dermatologist. Seborrheic dermatitis is a heterogenous condition involving Th1/Th17 inflammation and microbial skin and gut dysbiosis. Shared decision making is critical in determining whether and how to treat it. With the recent FDA approved therapies for seborrheic dermatitis, we now have the opportunity to look at this condition with fresh eyes.
References
-
- Gupta AK, Bluhm R, Cooper EA, Summerbell RC, Batra R. Seborrheic dermatitis. Dermatologic Clinics. 2003;21(3):401-412. doi: 10.1016/s0733-8635(03)00028-7.
- Naldi L, Rebora A. Seborrheic dermatitis. N Engl J Med. 2009;360(4):387-396. doi: 10.1056/NEJMcp0806464
- Tao R, Li R, Wang R. Skin microbiome alterations in seborrheic dermatitis and dandruff: A systematic review. Exp Dermatol. 2021;30(10):1546-1553.
- Dall’Oglio F, Nasca MR, Gerbino C, Micali G. An overview of the diagnosis and management of seborrheic dermatitis. Clin Cosmet Investig Dermatol. 2022;15:1537-1548.
- Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis: a comprehensive review. J Dermatolog Treat. 2019;30(2):158-169.
- Papp KA, Papp A, Dahmer B, Clark CS. Single-blind, randomized controlled trial evaluating the treatment of facial seborrheic dermatitis with hydrocortisone 1% ointment compared with tacrolimus 0.1% ointment in adults. J Am Acad Dermatol. 2012;67(1):e11-15
- Joly P, Tejedor I, Tetart F, et al. Tacrolimus 0.1% versus ciclopiroxolamine 1% for maintenance therapy in patients with severe facial seborrheic dermatitis: A multicenter, double-blind, randomized controlled study. J Am Acad Dermatol. 2021;84(5):1278-1284.
- Peña SM, Oak ASW, Smith AM, Mayo TT, Elewski BE. Topical crisaborole is an efficacious steroid-sparing agent for treating mild-to-moderate seborrhoeic dermatitis. J Eur Acad Dermatol Venereol. 2020;34(12):e809-e812.
- Abbas Z, Ghodsi SZ, Abedeni R. Effect of itraconazole on the quality of life in patients with moderate to severe seborrheic dermatitis: a randomized, placebo-controlled trial. Dermatol Pract Concept. 2016;6(3):11-16.
- Cömert A, Bekiroglu N, Gürbüz O, Ergun T. Efficacy of oral fluconazole in the treatment of seborrheic dermatitis: a placebo-controlled study. Am J Clin Dermatol. 2007;8(4):235-238.
- Blauvelt A, Draelos ZD, Stein Gold L, et al. Roflumilast foam 0.3% for adolescent and adult patients with seborrheic dermatitis: A randomized, double-blinded, vehicle-controlled, phase 3 trial. J Am Acad Dermatol. 2024;90(5):986-993.
This information was presented by Dr. Shawn Kwatra at the 2024 Skin of Color Update Conference, held September 13-15, 2024. The above highlights from his lecture were written and compiled by Dr. Osuoji.
Did you enjoy this article? Find more on Skin of Color Dermatology here.