JDD February 2024 Issue Highlights
 Explore the February 2024 issue of the Journal of Drugs in Dermatology! Discover groundbreaking research on topics such as novel treatments for melasma, advancements in psoriasis management, gene expression profile (GEP) testing for squamous cell carcinoma, pediatric vitiligo, and many more! Stay ahead of the curve with the latest insights from leading dermatology experts. Straight from the Editor …
Explore the February 2024 issue of the Journal of Drugs in Dermatology! Discover groundbreaking research on topics such as novel treatments for melasma, advancements in psoriasis management, gene expression profile (GEP) testing for squamous cell carcinoma, pediatric vitiligo, and many more! Stay ahead of the curve with the latest insights from leading dermatology experts. Straight from the Editor …
 Explore the February 2024 issue of the Journal of Drugs in Dermatology! Discover groundbreaking research on topics such as novel treatments for melasma, advancements in psoriasis management, gene expression profile (GEP) testing for squamous cell carcinoma, pediatric vitiligo, and many more! Stay ahead of the curve with the latest insights from leading dermatology experts. Straight from the Editor …
Explore the February 2024 issue of the Journal of Drugs in Dermatology! Discover groundbreaking research on topics such as novel treatments for melasma, advancements in psoriasis management, gene expression profile (GEP) testing for squamous cell carcinoma, pediatric vitiligo, and many more! Stay ahead of the curve with the latest insights from leading dermatology experts. Straight from the Editor … 

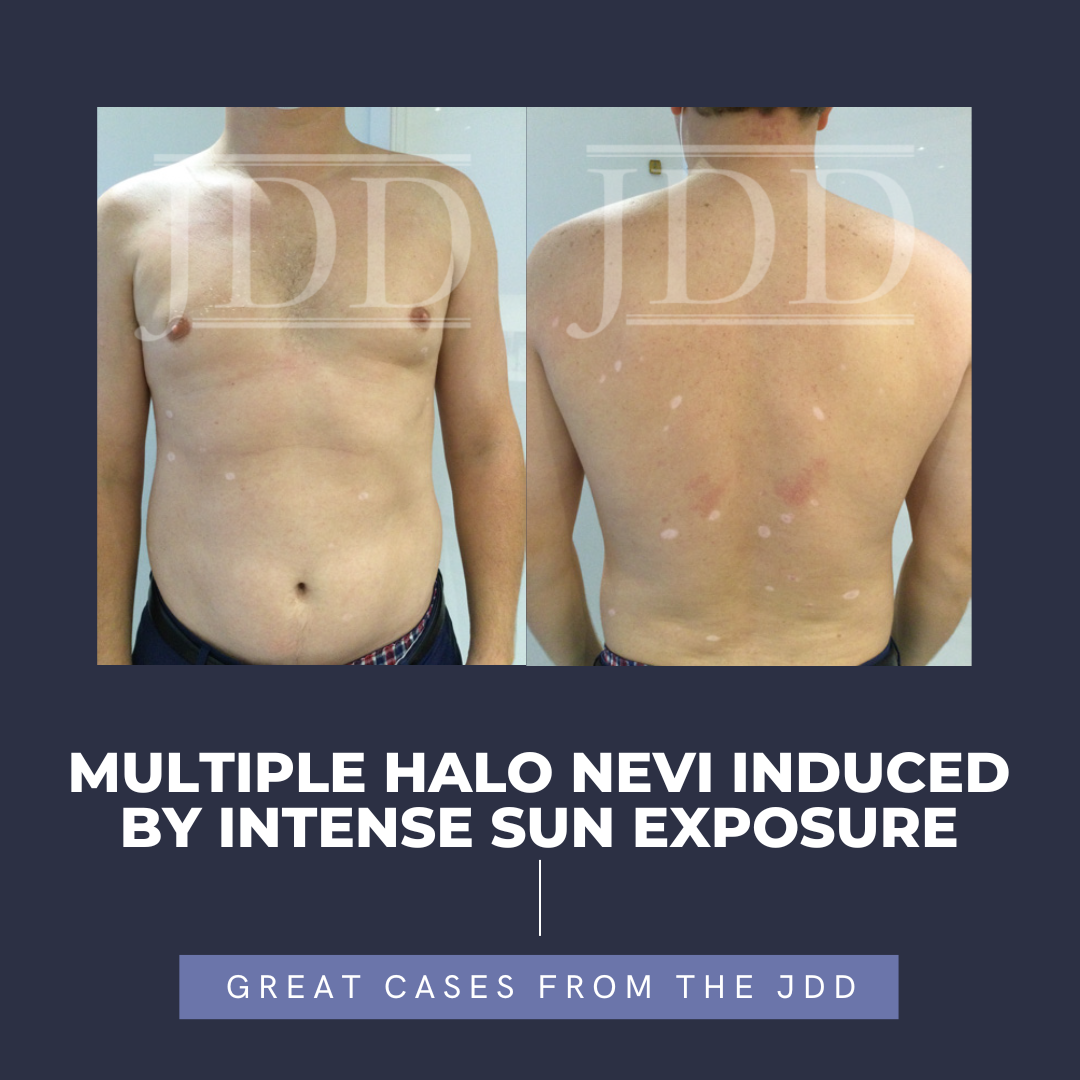 JDD authors present the case of a 38-year-old male who reported to their practice with multiple newly developed halos around 26 existing nevi on his trunk. The halo nevi developed after the patient, who lived in the northeast, spent 2 months on a lake in Alabama, with intense heat and sun exposure. This case is remarkable in that it points to ultraviolet exposure as one instigating factor in the …
JDD authors present the case of a 38-year-old male who reported to their practice with multiple newly developed halos around 26 existing nevi on his trunk. The halo nevi developed after the patient, who lived in the northeast, spent 2 months on a lake in Alabama, with intense heat and sun exposure. This case is remarkable in that it points to ultraviolet exposure as one instigating factor in the …  Media attention about oral minoxidil has impacted access to the hair loss drug in the Washington, D.C. area, according to a study published in the January issue of the Journal of Drugs in Dermatology. Researchers found shortages of the drug in D.C.-area pharmacies more than a year after an article about the treatment appeared in The New York Times.
To find out more about oral minoxidil access, …
Media attention about oral minoxidil has impacted access to the hair loss drug in the Washington, D.C. area, according to a study published in the January issue of the Journal of Drugs in Dermatology. Researchers found shortages of the drug in D.C.-area pharmacies more than a year after an article about the treatment appeared in The New York Times.
To find out more about oral minoxidil access, … 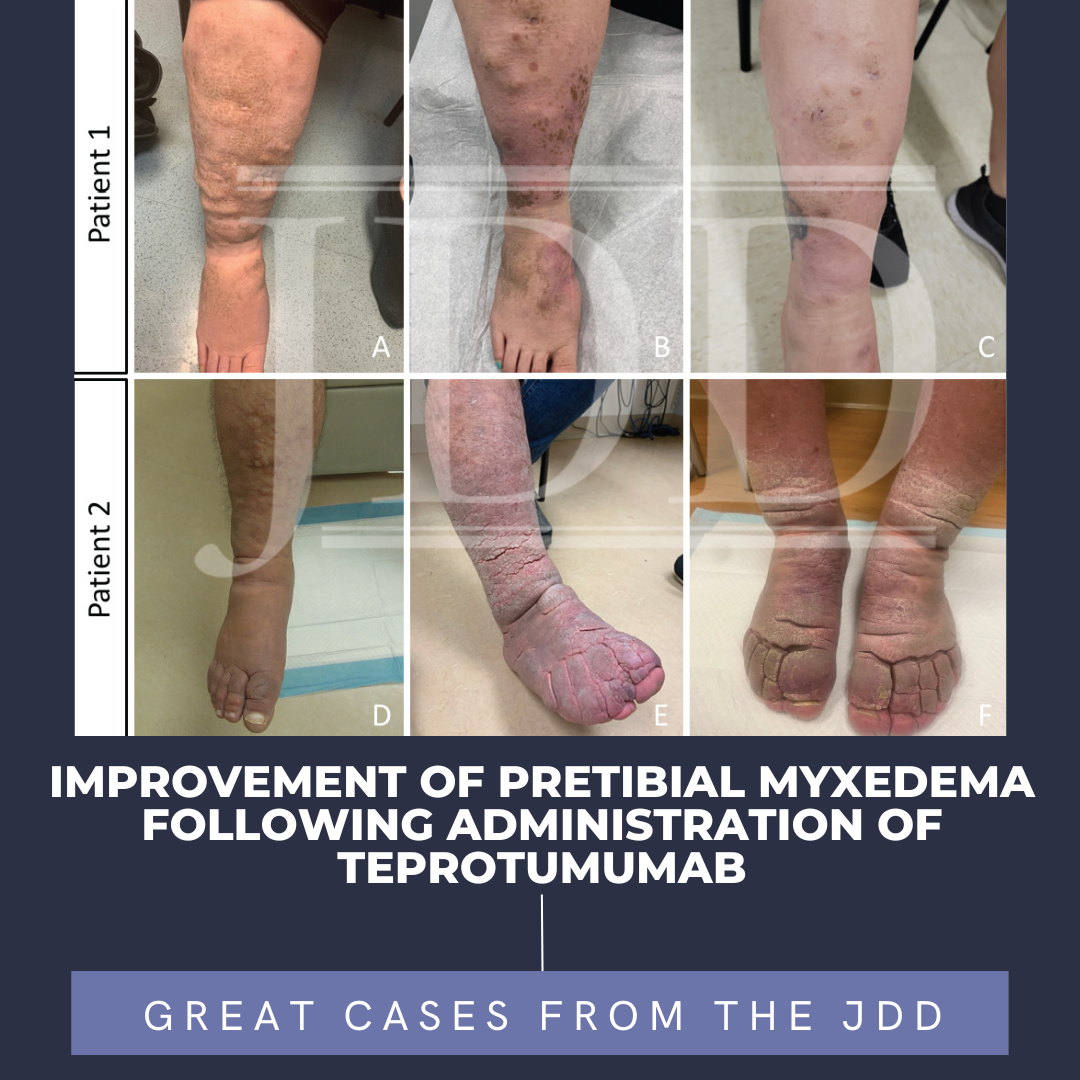 Pretibial myxedema (PTM) is a rare complication of Graves' disease. It is characterized by non-pitting edema with hyperpigmented hyperkeratotic papules and plaques on bilateral lower legs. Effective treatments for patients with PTM are lacking. The etiology of PTM is unknown; however, it may be similar to the mechanism of thyroid-associated ophthalmopathy (TAO). Activated fibroblasts produce infla …
Pretibial myxedema (PTM) is a rare complication of Graves' disease. It is characterized by non-pitting edema with hyperpigmented hyperkeratotic papules and plaques on bilateral lower legs. Effective treatments for patients with PTM are lacking. The etiology of PTM is unknown; however, it may be similar to the mechanism of thyroid-associated ophthalmopathy (TAO). Activated fibroblasts produce infla …  The Journal of Drugs in Dermatology welcomes 2024 with a strong line up of original articles, case reports, brief communications, and letters to the editor. Straight from the Editor's desk, we share this month's issue highlights!
ORIGINAL ARTICLES
Current Landscape of Hyaluronic Acid Filler Use in the United States shares the results of a comprehensive search of all the FDA approved dermal fill …
The Journal of Drugs in Dermatology welcomes 2024 with a strong line up of original articles, case reports, brief communications, and letters to the editor. Straight from the Editor's desk, we share this month's issue highlights!
ORIGINAL ARTICLES
Current Landscape of Hyaluronic Acid Filler Use in the United States shares the results of a comprehensive search of all the FDA approved dermal fill …