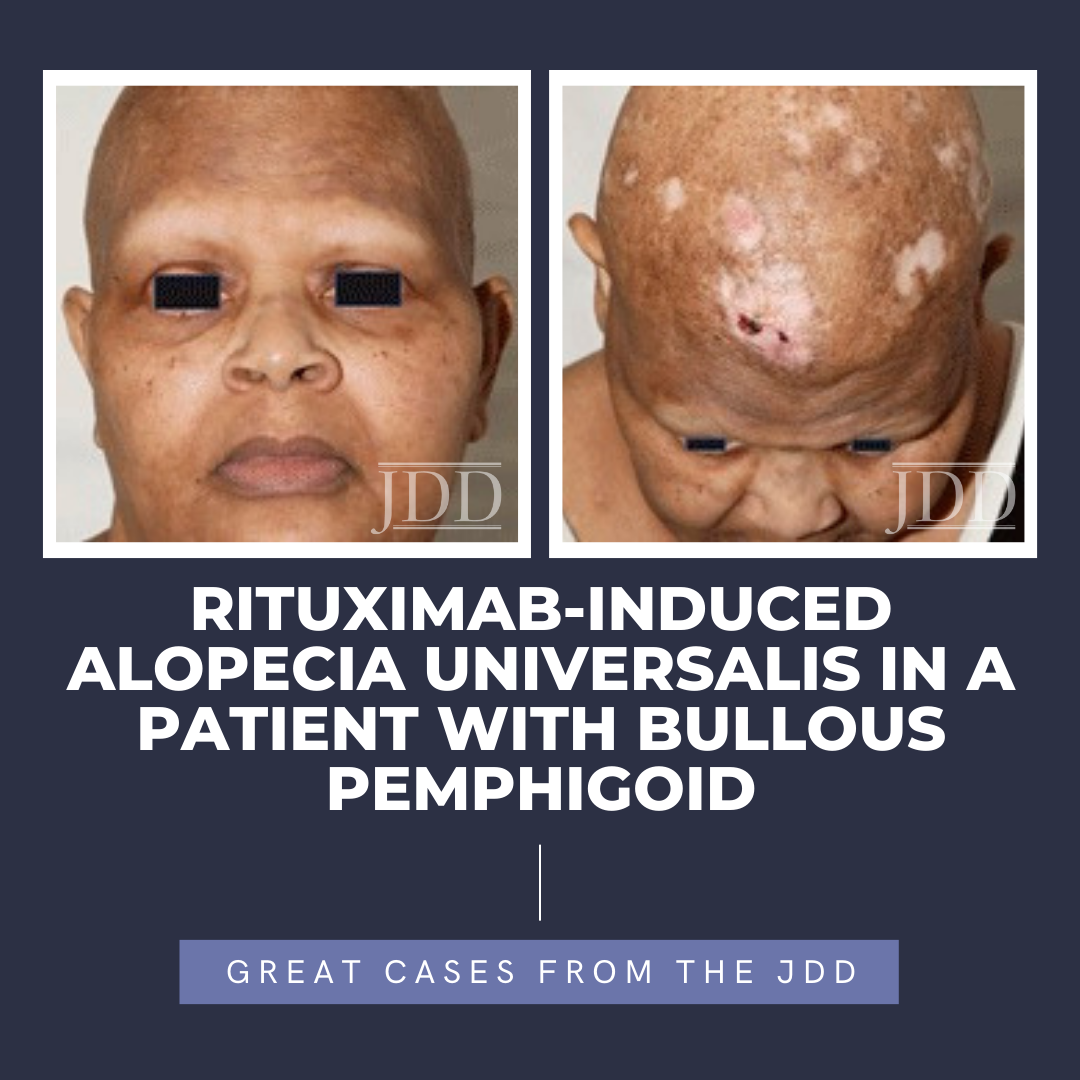
Alopecia areata is a CD8+ T-lymphocyte driven autoimmune disorder leading to reversible hair loss. While most commonly presenting as isolated well-demarcated non-cicatricial alopecic patches on the scalp, subtypes of alopecia areata include alopecia totalis with loss of all scalp hair and alopecia universalis with complete loss of all body hair. Although primarily an idiopathic condition, several triggers, including medications, have been reported in the literature. To the best of the authors’ knowledge, they present the first reported case of rituximab, a chimeric anti-CD20 receptor monoclonal antibody, inducing alopecia universalis on two separate occasions during the treatment of bullous pemphigoid in a 55-year-old female. While the exact mechanism driving this association remains unclear, greater insights into the pathophysiology of alopecia areata/universalis may help to further explain this association and provide greater insight into other possible therapeutic options.
CASE PRESENTATION
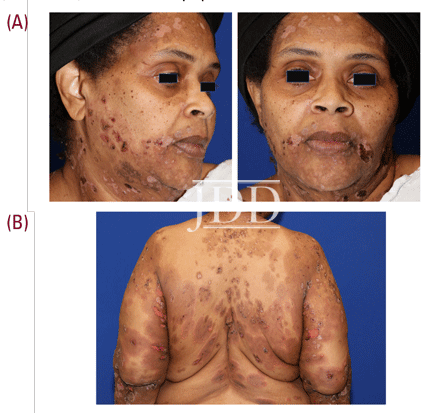
A 55-year-old female with a history of hypertension and discoid lupus presented to dermatology clinic with widespread urticated tense bullae and crusted erosions present for several months (Figure 1), consistent with bullous pemphigoid on further tissue and serological evaluation. Treatment was initiated with a prolonged prednisone taper, starting at 1 mg/kg, in conjunction with mycophenolate mofetil at a dose of 1 g daily. Although well-controlled initially, breakthrough blistering was noted approximately 6 months into the treatment course. At that time, the risks and benefits of the off-label use of rituximab for bullous pemphigoid was discussed,1-2 and patient elected to proceed with rituximab treatment.
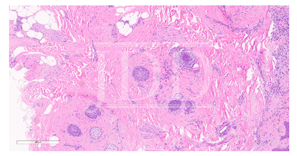
The patient presented in follow up one month after completing two loading doses of rituximab 1,000 mg IV spaced two weeks apart with subsequent complete resolution of blistering and pruritus. Over the next few months, she developed progressive and eventually complete non-scarring alopecia involving the scalp, eyebrows, and eyelashes. Of note, our patient did have a background of previous scattered atrophic cicatricial alopecic plaques involving the scalp in areas of discoid lupus involvement (Figure 2). Biopsy obtained from an area of scarring on the scalp demonstrated features consistent with her known previous diagnosis of discoid lupus, with a negative evaluation for systemic lupus. Biopsy obtained from a non-scarring area on the scalp demonstrated histopathologic features diagnostic of alopecia areata (Figure 3). Treatment was initiated with clobetasol 0.05% ointment twice daily to the scalp for 2 weeks on, 2 weeks off.
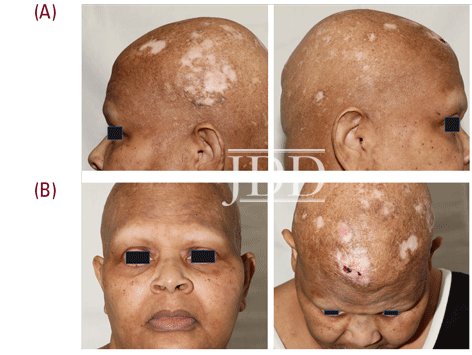
At the 6-month time point from first rituximab infusion, hair regrowth was noted involving affected non-scarring areas, including eyebrows, eyelashes, and scalp. Since causality related to rituximab had not been established at this time point and alopecia areata/universalis had otherwise not been reported in the literature in relation to rituximab administration, our patient elected to proceed with her third 1,000 mg IV rituximab infusion. Several weeks following this, she again noted complete non-scarring alopecia involving the scalp, eyebrows, and eyelashes, with subsequent hair regrowth, noted several months later.
6b75aa
DISCUSSION
CONCLUSION
REFERENCES
2. Bilgiç Temel A, Bassorgun CI, Akman-KarakaÅŸ A, Alpsoy E, Uzun S. Successful Treatment of a Bullous Pemphigoid Patient with Rituximab Who Was Refractory to Corticosteroid and Omalizumab Treatments. Case Rep Dermatol. 2017;9(1):38-44.
3. Wang EHC, Yu M, Breitkopf T, Akhoundsadegh N, Wang X, Shi FT, Leung G, Dutz JP, Shapiro J, McElwee KJ. Identification of Autoantigen Epitopes in Alopecia Areata. J Invest Dermatol. 2016;136(8):1617-1626. doi:10.1016/j.jid.2016.04.004.
4. Juhasz M, Mesinkovska NA. Are Preferred Scalp Locations for Alopecia Areata Patches a Clue to Neuronal Etiology? Skin Appendage Disord. 2019;5(5):283- 287. doi: 10.1159/000497392.
5. Lepe K, Zito PM. Alopecia Areata. [Updated 2021 Aug 27]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021 Jan. Available from: https:// www.ncbi.nlm.nih.gov/books/NBK537000/
6. Murad A, Maguire J, Bergfeld W. Drug-induced alopecia areata? Clin Exp Dermatol. 2021;46(2):363-366. doi: 10.1111/ced.14381.
7. Anuoluwapo R Oke, Steven Young-Min. EP06 Successful treatment of alopecia universalis with rituximab therapy. Rheumatology. 2020; Volume 59(2):133-134. doi.org: 10.1093/rheumatology/keaa109.005
8. Rituxan [package insert]. South San Francisco, CA: Biogen and Genentech USA, Inc.
SOURCE
Content and images used with permission from the Journal of Drugs in Dermatology.
Adapted from original article for length and style.
Did you enjoy this JDD case report? You can find more here.