INTRODUCTION
Melanoma accounts for only 1% of all skin cancers but is responsible for most skin cancer-related deaths.1 Acral and mucosal melanoma (AMM) accounts for only 4% of all new melanomas but is associated with poorer prognosis.2 Patients of color are disproportionately affected by AMM. Elucidating the clinical, genetic, and environmental features of AMM can guide advancements in their prevention and treatment. Herein we discuss six ethnically diverse cases.
Case Presentations
Case 1
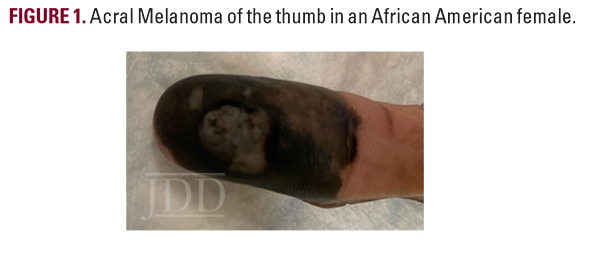
A 48-year-old African American woman presented for evaluation of a black spot on her right thumb that had enlarged over seven years (Figure 1). The patient’s past medical history was significant for multiple stab wounds to the right arm over 25 years ago. Physical examination revealed a hyperpigmented, ulcerating plaque on the volar aspect of the right thumb with a central punctum draining clear fluid. Histopathology confirmed the diagnosis revealing Breslow thickness of 2.7 mm, Clark IV (Stage cT3aN0) melanoma. Right axillary sentinel lymph node biopsy demonstrated no signs of lymph node metastasis. The lesion was definitively treated with right thumb amputation and close monitoring for disease recurrence.
Case 2
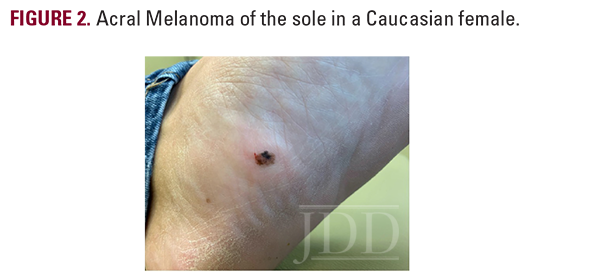
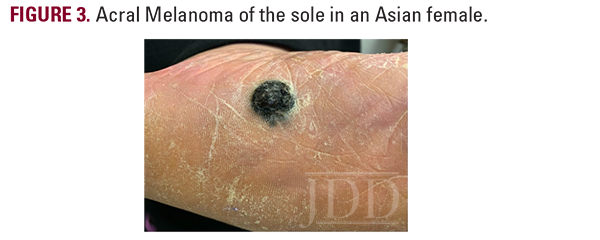
A 43-year-old Caucasian woman presented with a pigmented lesion on the left sole. The patient reported a change in color and morphology of the lesion over time, but denied any associated pain, bleeding, or lymphadenopathy. Past medical history was significant for hypertension and the use of tanning beds. A physical exam revealed an irregularly shaped black and brown papule on the plantar aspect of the left foot (Figure 2).
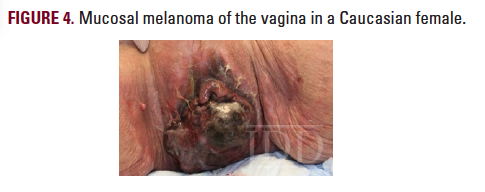
for MART-1, CD117, Vimentin, and S100, supporting a diagnosis of vaginal primary malignant melanoma. Inguinal lymph node biopsy showed neoplastic cells with nuclear hyperchromasia, enlargement, and pleomorphism.
DISCUSSION
The World Health Organization’s melanoma classification system currently recognizes 9 melanoma subtypes, of which 6 are not associated with cumulative solar damage (CSD).3 Included in this non-CSD division are AMM, with distinct clinical presentations, pathogenetic features, and epidemiologic characteristics separating each from other subtypes.
Acral melanoma occurs on the volar aspects of the hands and feet, as well as the nail beds.3 These lesions usually begin as a patch, enlarge radially, and may form plaques that result in epidermal thickening.3 However, given the thick stratum corneum on acral surfaces, the lesions often remain flat during radial growth.3 The vertical growth phase results in ulceration or nodule formation.3 Diagnosis of acral lesions often occurs at advanced stages leading to poor prognosis.3
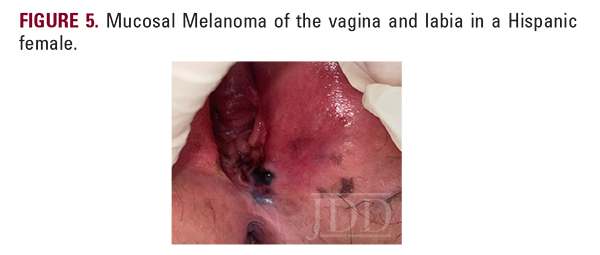
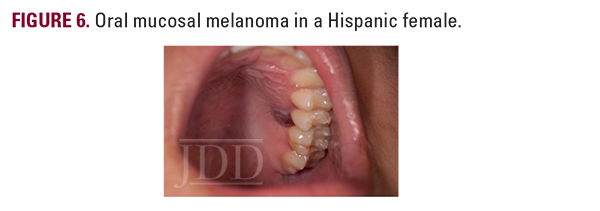
Mucosal melanoma occurs within a mucous membrane, primarily in genital sites, oral/nasal cavities, and the conjunctiva.3 Detection is difficult given the non-visible sites and 40% of cases being amelanotic.4 Therefore, these lesions commonly present as bulky, invasive tumors.3
The role of UV radiation in the mutagenesis of classic cutaneous melanoma is well established, but the pathogenesis of AMM is poorly understood.5 There is some evidence that previous trauma and human herpes virus DNA may play a role in the development of some acral and mucosal melanomas, respectively.3,6 Though 40 to 60% of cutaneous melanomas have activating BRAF mutations, mutations in KIT, NRAS, and CCND1 appear to play a more significant role in the mutagenesis of AMM.3,7 TERT translocations have been noted in 41% of cases.3
AMM have distinct epidemiological characteristics. AMM occurs at an approximately equal rate among all races. However, as patients of color are less prone to melanoma developing in sunexposed sites, a greater proportion of total melanoma in these patients is AMM.3 Difficulty in recognizing these lesions in skin of color likely contributes to advanced stage at diagnosis and poorer prognosis.
Review studies aimed at investigating the different subtypes of melanoma and their associated risk factors are underway.8 In the meantime, encouraging regular multidisciplinary care visits can aid in early diagnosis and decrease disease burden. Performing a thorough physical at routine intervals, including examination of commonly overlooked areas such as nasal/oral cavities, hands/feet, and genitalia is essential.
CONCLUSION
DISCLOSURES
REFERENCES
-
- American Cancer Society. Cancer Facts & Figures 2021. Atlanta: American Cancer Society; 2021. https://www.cancer.org/content/dam/cancer-org/ research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures- 2021.pdf
- Hall KH, Rapini RP. Acral Lentiginous Melanoma. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Available at: http://www. ncbi.nlm.nih.gov/books/NBK559113. Accessed Sep 26, 2021.
- Elder DE, Bastian BC, Cree IA, et al. The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: detailed analysis of 9 distinct subtypes defined by their evolutionary pathway. Arch Pathol Lab Med. 2020;144(4):500-22.
- Carvajal RD, Spencer SA, Lydiatt W. Mucosal melanoma: a clinically and biologically unique disease entity. J Natl Compr Cancer Netw JNCCN. 2012;10(3):345-56.
- Whiteman DC, Pavan WJ, Bastian BC. The melanomas: a synthesis of epidemiological, clinical, histopathological, genetic, and biological aspects, supporting distinct subtypes, causal pathways, and cells of origin. Pigment Cell Melanoma Res. 2011;24(5):879-97.
- Lundberg R, Brytting M, Dahlgren L, et al. Human herpes virus DNA is rarely detected in non-UV light-associated primary malignant melanomas of mucous membranes. Anticancer Res. 2006;26(5B):3627-31.
- Chacón M, Pfluger Y, Angel M, et al. Uncommon subtypes of malignant melanomas: a review based on clinical and molecular perspectives. Cancers. 2020;12(9):E2362.
- Puyana C, Rajkumar J, Tsoukas MM. Primary tumor location predicts survival in melanoma: a retrospective cohort study of 239,257 melanoma patients. Skinmed. 2021;19(4):288-96.
SOURCE
Burningham, Kevin M., et al. “Case Series of Acral and Mucosal Melanoma in a Diverse Patient Population.” Journal of drugs in dermatology: JDD 23.8 (2024): 683-685.
Content and images used with permission from the Journal of Drugs in Dermatology.
Adapted from original article for length and style.
Did you enjoy this JDD case report? You can find more here.