ABSTRACT
INTRODUCTION
The use of soft tissue fillers is an increasingly popular means of rejuvenation; it was the second most common minimally invasive cosmetic procedure worldwide in 2020.1 Although fillers have a favorable safety profile,2 adverse events may still occur. One such event is the occurrence of delayed onset nodules. Although more common with permanent fillers such as polymethylmethacrylate or silicone, nodules have also been reported with non-permanent hyaluronic acid fillers.3,4
Historically, the risk of delayed onset nodules in the hands of a well-trained injector is low2,4-6 with the incidence for granulomatous reactions ranging from 0.02%-0.4%.7 However, there is a reported increase in nodule formation with the use of newer fillers with proprietary cross-linking technology.8-11
CASE REPORT
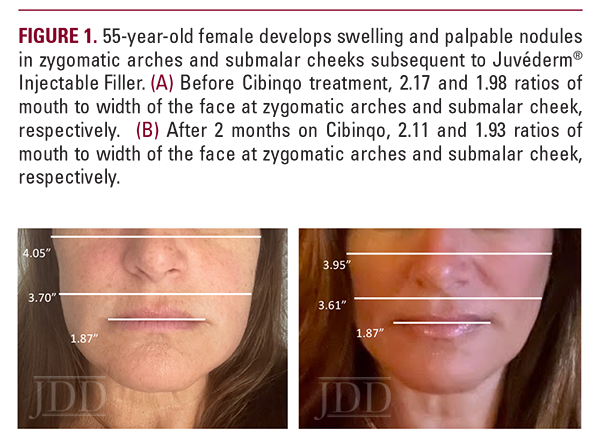
A 55-year-old woman presented with a chief complaint of swelling over her cheeks and jawline 6 weeks after hyaluronic acid filler injections (Juvederm Voluma) to her zygomatic arches. The patient reported previous hyaluronic acid filler injections without complications. Past medical history included atopic dermatitis particularly affecting her face, as well as Hashimoto’s thyroiditis. Prior to consulting dermatology, the patient underwent three courses of oral antibiotics (cephalexin, amoxicillin clavulanate, and clarithromycin) as well as two separate week-long courses of a methylprednisolone taper. She reported rapid improvement during steroid therapy but with rebound swelling upon completion of each taper. Concomitantly, five courses of hyaluronidase injections were also attempted, which softened and decreased the size of some but not all of the nodules. Oral antihistamines were of no benefit.
On physical examination, the patient had erythematous patches on bilateral eyelids and malar cheeks, mild swelling of the zygomatic arches and lower cheeks, and multiple firm, palpable nodules of varying sizes over the upper, mid, and lower cheeks. The patient also had dermographism at the time of her visit, and eyelid swelling.
DISCUSSION
Late-onset nodules post-filler injections are an uncommon and unpredictable complication. The pathophysiology for this phenomenon remains unclear — multiple mechanisms have been implicated including protein impurities left over from the bacterial fermentation process9 and biofilm formation.7,13 Recently, it has been proposed that the breakdown of the cross-linking components used to stabilize the filler may lead to an immunologic reaction and subsequent granuloma formation.8,10,11,14
Although nodules may resolve over time without intervention, the typical standard of care for persistent nodules includes oral and intralesional steroids, antibiotic therapy, and hyaluronidase injections. Other novel measures to manage nodules include the use of lasers15 as well as energy-based devices.16 Definitive management of recalcitrant nodules includes surgical removal or incision and drainage.2,7
CONCLUSION
ACKNOWLEDGEMENT
REFERENCES
-
- ISAPS-Global-Survey_2020.pdf. Accessed August 17, 2022. https://www. isaps.org/wp-content/uploads/2022/01/ISAPS-Global-Survey_2020.pdf
- Signorini M, Liew S, Sundaram H, et al. Global aesthetics consensus: avoidance and management of complications from hyaluronic acid fillers: evidence- and opinion-based review and consensus recommendations. Plast Reconstr Surg. 2016;137(6):961-971. doi:10.1097/PRS.0000000000002184
- Humphrey et al. – 2020 – Retrospective review of delayed adverse events sec.pdf. Accessed August 17, 2022. https://www.jaad.org/action/showPdf?p ii=S0190-9622%2820%2930152-3
- Ledon JA, Savas JA, Yang S, Franca K, Camacho I, Nouri K. Inflammatory nodules following soft tissue filler use: a review of causative agents, pathology and treatment options. Am J Clin Dermatol. 2013;14(5):401-411. doi:10.1007/s40257-013-0043-7
- Andre P. Evaluation of the safety of a non-animal stabilized hyaluronic acid (NASHA – Q-Medical, Sweden) in European countries: a retrospective study from 1997 to 2001. J Eur Acad Dermatol Venereol. 2004;18(4):422-425. doi:10.1111/j.1468-3083.2004.00934.x
- Modarressi A, Nizet C, Lombardi T. Granulomas and nongranulomatous nodules after filler injection: Different complications require different treatments. J Plast Reconstr Aesthet Surg. 2020;73(11):2010-2015. doi:10.1016/j.bjps.2020.08.012
- Abduljabbar MH, Basendwh MA. Complications of hyaluronic acid fillers and their managements. J Dermatol Dermatol Surg. 2016;20(2):100-106. doi:10.1016/j.jdds.2016.01.001
- Sadeghpour M, Quatrano NA, Bonati LM, Arndt KA, Dover JS, Kaminer MS. Delayed-onset nodules to differentially crosslinked hyaluronic acids: comparative incidence and risk assessment. Dermatol Surg. 2019;45(8):1085-1094. doi:10.1097/DSS.0000000000001814
- Artzi O, Loizides C, Verner I, Landau M. Resistant and recurrent late reaction to hyaluronic acid-based gel. Dermatol Surg. 2016;42(1):31-37. doi:10.1097/ DSS.0000000000000562
- Beleznay K, Carruthers JDA, Carruthers A, Mummert ME, Humphrey S. Delayed-onset nodules secondary to a smooth cohesive 20 mg/mL hyaluronic acid filler: cause and management. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2015;41(8):929-939. doi:10.1097/DSS.0000000000000418
- Humphrey S, Jones DH, Carruthers JD, et al. Retrospective review of delayed adverse events secondary to treatment with a smooth, cohesive 20-mg/mL hyaluronic acid filler in 4500 patients. J Am Acad Dermatol. 2020;83(1):86-95. doi:10.1016/j.jaad.2020.01.066
- Cibinqo (abrocitinib) [prescribing information]. New York, NY: Pfizer Labs; January 2022. Published online January 2022. Accessed September 9, 2022.
- Alhede M, Er O, Eickhardt S, et al. Bacterial biofilm formation and treatment in soft tissue fillers. Pathog Dis. 2014;70(3):339-346. doi:10.1111/2049- 632X.12139
- Rivers JK. Incidence and treatment of delayed-onset nodules after VYC filler injections to 2139 patients at a single Canadian clinic. J Cosmet Dermatol. 2022;21(6):2379-2386. doi:10.1111/jocd.15013
- Zaccaria G, Cassuto D, Baccarani A, Lusetti IL, Santis GD. Filler-induced complications of the lips: 10 years experience with intralesional laser treatment and refinements. J Plast Reconstr Aesthet Surg. 2022;75(3):1215- 1223. doi:10.1016/j.bjps.2021.11.042
- Ostezan L, Peck J. Radial Sound (Shockwave) Therapy resolves delayed-onset nodules following injection of hyaluronic acid dermal filler: a case study. J Clin Aesthetic Dermatol. 2021;14(12 Suppl 1):S15-S17.
- Talty R, Damsky W, King B. Treatment of cutaneous sarcoidosis with tofacitinib: A case report and review of evidence for Janus kinase inhibition in sarcoidosis. JAAD Case Rep. 2021;16:62-64. doi:10.1016/j.jdcr.2021.08.012
- Damsky W, Thakral D, McGeary MK, Leventhal J, Galan A, King B. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82(3):612-621. doi:10.1016/j. jaad.2019.05.098
- Damsky W, Wang A, Kim DJ, et al. Inhibition of type 1 immunity with tofacitinib is associated with marked improvement in longstanding sarcoidosis. Nat Commun. 2022 Jun 6;13(1):3140. doi: 10.1038/s41467-022- 30615-x
- Wang A, Rahman NT, McGeary MK, et al. Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021 May;147(5):1795-1809. doi: 10.1016/j. jaci.2020.10.012
SOURCE
Lopez, Miyahra Haniko P., et al. “Post-Hyaluronic Acid Filler Reaction Treated With Abrocitinib: A Case Report.” Journal of Drugs in Dermatology: JDD 23.1 (2024): 1355-1356.
Adapted from original article for length and style.