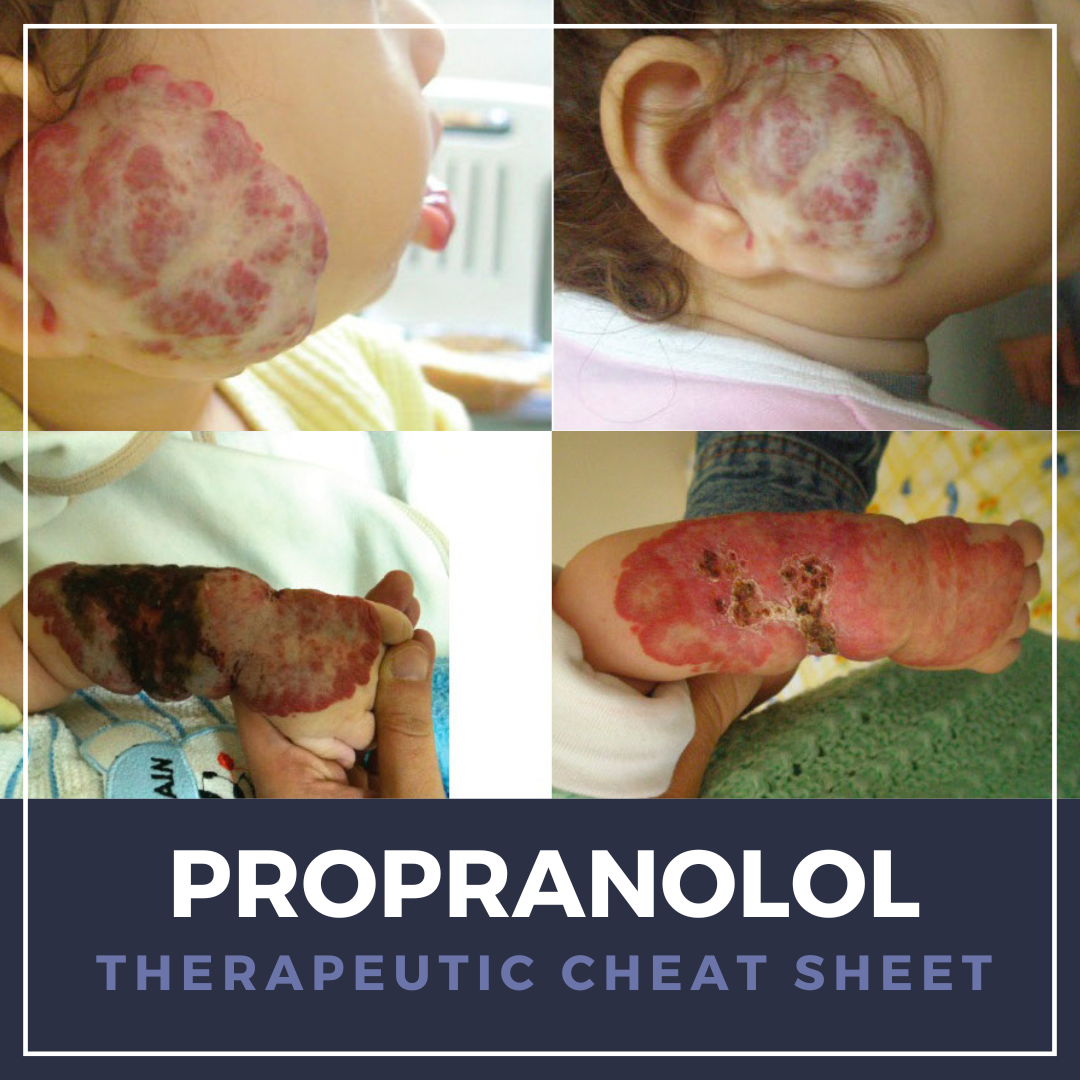
Propranolol, a nonselective β-adrenergic blocker introduced in the 1960s, remains a cornerstone therapy in cardiology for hypertension, angina, and migraine prophylaxis. It has since gained transformative use in dermatology following the serendipitous discovery of its efficacy in infantile hemangiomas (IH), becoming the first FDA-approved systemic therapy for proliferating IH in 2014 given its vasoconstrictive and anti-angiogenic properties.1,2
Beyond IH, propranolol has been employed off-label for a range of dermatologic conditions, including rosacea-associated flushing, adrenergic urticaria, hyperhidrosis, trichodynia, and other vascular tumors. While generally well-tolerated, clinicians must be mindful of risks such as hypoglycemia, bradycardia, hypotension, and bronchospasm, particularly in infants and patients with comorbid cardiopulmonary disease.1-7
We continue our “Therapeutic Cheat Sheet” series with a focused review of propranolol’s dermatologic applications, safety considerations, and practical prescribing pearls.
Propranolol Therapeutic Cheat Sheet
Compiled by: Nathaniel Lampley, MD | Reviewed by: Adam Friedman, MD
TRADE NAME1,9
Hemangeol®, Inderal®, InnoPran XL®, Detensol®, Novo-Pranol®, Deralin®, and Cardinol®
MECHANISM OF ACTION2
Nonselective β-adrenergic blocker:
-
- Competitive blockade of β1- and β2-receptors, leading to decreased cardiac output and inhibition of smooth muscle relaxation and glycogenolysis.
IH:
-
- Induces vasoconstriction, reduces angiogenesis through inhibition of vascular endothelial growth factor, and promotes endothelial cell apoptosis.
FDA-APPROVED FOR3,4
-
- Proliferating IH requiring systemic therapy (Hemangeol)
- Hypertension
- Angina pectoris due to coronary atherosclerosis
- Migraine prophylaxis
- Hypertrophic subaortic stenosis
OFF-LABEL DERMATOLOGIC USES2,6-8
-
- Rosacea (flushing)
- Hyperhidrosis
- Adrenergic urticaria
- Other vascular malformations (e.g. arteriovenous malformation, capillary malformation, venous malformation) and tumors (e.g. kaposiform hemangioendothelioma, angiosarcoma)
- Trichodynia
DOSING
Infantile Hemangioma2,3
-
- Recommendations from FDA label:
- Start treatment between 5 weeks and 5 months with 0.6 mg/kg BID.
- Increase to 1.1 mg/kg after 1 week
- Increase to a maintenance dose of 1.7 mg/kg after 2 weeks
- Recommendations from published literature:
- Start 1 mg/kg/day PO divided BID; increase to 2-3 mg/kg/day divided BID with feeds.
- Given at least 9 hours apart with or after feeding. Hold during poor intake, vomiting, or intercurrent illness.
- Recommendations from FDA label:
Rosacea / Flushing6
-
- PRN: 10-40 mg PO QD-TID
WARNINGS AND PRECAUTIONS3
-
- Hypoglycemia: Administer during or after feeding. Do not use in patients who are not able to feed or are vomiting.
- Bradycardia and hypotension
- Bronchospasm: Avoid use in patients with asthma or lower respiratory infection.
- Increased risk of stroke in PHACE syndrome
- Hypersensitivity: Interferes with epinephrine used to treat anaphylaxis.
SIDE EFFECTS3
-
- Common: sleep disorders, aggravated respiratory tract infections associated with cough and fever, diarrhea, vomiting
- Rare (incidence of <1%): Second degree AV block, urticaria, alopecia, hypoglycemia, sinus bradycardia
- Side effects reported postmarketing: agranulocytosis, hallucination, purpura, psoriasiform dermatitis
DRUG INTERACTIONS3,10
-
- Corticosteroids: Increased risk of hypoglycemia
- CYP2D6
- Inhibitors ↑ propranolol plasma concentration (e.g. paroxetine, fluoxetine, fluoxetine, quinidine)
- Inducers ↓ propranolol plasma concentration
- CYP1A2
- Inhibitors ↑ propranolol plasma concentration (e.g. fluvoxamine)
- Inducers ↓ propranolol plasma concentration (e.g. phenytoin, phenobarbital, omeprazole)
- CYP2C19
- Inhibitors ↑ propranolol plasma concentration (e.g. loratidine, fluvoxamine)
- Inducers ↓ propranolol plasma concentration (e.g. rifampin)
CONTRAINDICATIONS3
-
- Premature infants with corrected age <5 weeks
- Infants weighing less than 2 kg
- Known hypersensitivity to propranolol or excipients
- Asthma or history of bronchospasm
- Sinus bradycardia (<80 beats per minute)
- >1st-degree AV block
- Decompensated heart failure
- Blood pressure <50/30 mmHg
- Pheochromocytoma
- Inderal: Bronchial asthma, Sinus bradycardia, >1st-degree AV block, Cardiogenic shock, Congestive heart failure
PREGNANCY AND BREASTFEEDING3
-
- Pregnancy: Animal studies have shown that propranolol exposure before and during pregnancy, as well as during early postnatal development, had no adverse effects on fertility, reproductive parameters, or reproductive development even at doses exceeding those used in humans.
- Breastfeeding: Propranolol is excreted in breast milk, and available data suggest no adverse effects on nursing infants.
MONITORING3
-
- Prior to initiating medication, recommend: baseline weight, HR, BP; feeding history, cardiac history, respiratory history.
- When initiating medication: monitor heart rate and blood pressure for 2 hours after initiation or dose increases.
- After initiating medication, monitor ongoing for signs of hypoglycemia, bradycardia, hypotension.

FURTHER READING
If you would like to learn more about propranolol, check out the following articles in the Journal of Drugs in Dermatology:
Vessel-Targeting Therapies for the Management of Rosacea: A Review of Current Evidence
I. Vidal, N. Menta, A. Friedman
ABSTRACT
The authors review vessel-targeting therapies, including β-blockers, and their usage in the management of rosacea.
The Potential Efficacy of β-Blockers on Melanoma Survival: A Narrative Review
M. Goldust, Z. Apalla, J.C. Szepietowski, M. Gupta, T. Lotti
ABSTRACT
Melanoma is a common tumor accounting for around 3–5% of all cutaneous malignancies with worldwide increasing incidence. It is still associated with significant mortality despite the breakthrough of new innovative therapies within the last decade. A wide variety of treatment modalities is currently used for the management of melanoma, ranging from surgical excision of primary melanoma to adju-vant and palliative treatment with target molecules, including BRAF and MEK inhibitors, and immune checkpoint inhibitors. β-blockers have recently demonstrated in preclinical and clinical studies to reduce recurrence and to correlate with better overall survival in meta-static melanoma as an additional supportive treatment option, owing to their anti-tumor potential. Further investigation regarding their efficacy and safety profile is needed, since there are only few studies in the literature on this topic. Our aim is to evaluate the role and current status of β-blockers in melanoma management. The literature research includes peer-reviewed articles (clinical trials or scien-tific reviews). Studies were identified by searching electronic databases (MEDLINE and PubMed) till May 2020 and reference lists of respective articles. Only articles published in English language were included.
Dany
ABSTRACT
Pyogenic granuloma (PG) is an acquired vascular growth on the skin and mucous membranes. Even though PG is a benign tumor, treatment is required due to associated risk of ulceration and bleeding, cosmetic concerns, and the low likelihood of spontaneous regression. Treatment entails excisional surgery, cryotherapy, or electrocautery; recurrence however is a major problem. Beta-blockers became an attractive option for the treatment of vascular growths after it got approved for infantile hemangioma. PG was found to express beta adrenergic receptors, similarly to infantile hemangioma. Several publications have reported the use of oral and topical beta blockers such as timolol, propranolol, and betaxolol for the treatment of PG. In this study, we summarized the literature with regards to the effectiveness of topical beta blockers for the treatment of PG, and discussed all published case reports, case series, and open-label single arm trials.
REFERENCES
-
- Fernandez Faith E, Shah SD, Braun M, et al. Paradigm shift in the management of infantile hemangiomas in the beta-blocker era: A retrospective cohort study. J Am Acad Dermatol. Published online July 12, 2025. doi:10.1016/j.jaad.2025.07.017
- Oberlin KE. Expanding uses of propranolol in dermatology. Cutis. 2017;99(4):E17-E19.
- U.S. FDA. HEMANGEOL® (propranolol hydrochloride) [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/205410s005lbl.pdf
- U.S. FDA. PROPRANOLOL HYDROCHLORIDE EXTENDED RELEASE
- CAPSULES [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/078065lbl.pdf
- Logger JGM, Olydam JI, Driessen RJB. Use of beta-blockers for rosacea-associated facial erythema and flushing: A systematic review and update on proposed mode of action. J Am Acad Dermatol. 2020;83(4):1088-1097. doi:10.1016/j.jaad.2020.04.129
- Goss JA, Konczyk DJ, Alomari MH, Maclellan RA, Greene AK. Propranolol Treatment of Vascular Anomalies Other Than Infantile Hemangioma. J Craniofac Surg. 2017;28(8):2001-2003. doi:10.1097/SCS.0000000000004166
- Rodriguez-Tamez G, Imbernon-Moya A, Saceda-Corralo D, Vano-Galvan S. [Translated article] Dermatology Update on the Challenging Trichodynia. Actas Dermosifiliogr. Published online July 19, 2025. doi:10.1016/j.ad.2025.07.006
- Propranolol. In: MotherToBaby | Fact Sheets. Brentwood (TN): Organization of Teratology Information Specialists (OTIS); September 2023.
- U.S. FDA. Drug Development and Drug Interactions | Table of Substrates, Inhibitors and Inducers. https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers
Did you enjoy this Therapeutic Cheat Sheet? You can find more here.