INTRODUCTION
CASE
DISCUSSION
The clinical manifestations of epoprostenol, a synthetic prostacyclin (PGI2), include vasodilation, inhibition of smooth muscle growth, and inhibition of platelet aggregation. It is commonly prescribed as a continuous IV infusion for severe PAH. As a last-resort medication, it is titrated based on symptoms, hemodynamic compromise, and adverse events. Dose-limiting side effects include headache, hypotension, flushing, arthralgia, and myalgia.2
The exact number of patients experiencing cutaneous adverse events remains relatively unknown due to the low prevalence of PAH. Flushing has been reported in as many as 58% of patients following epoprostenol use.3 Other cutaneous presentations include erythroderma,1,4 generalized blanching
erythema, palpable purpura, brawny discoloration, capillaritis, skin ulceration, eczematous dermatitis/rash/urticaria, and moderate pitting edema.2 The prostacyclin-IP receptor pathway has been implicated in nociceptive signal transmission, and lower extremity pain and swelling are common, though often successfully relieved with gabapentin.5
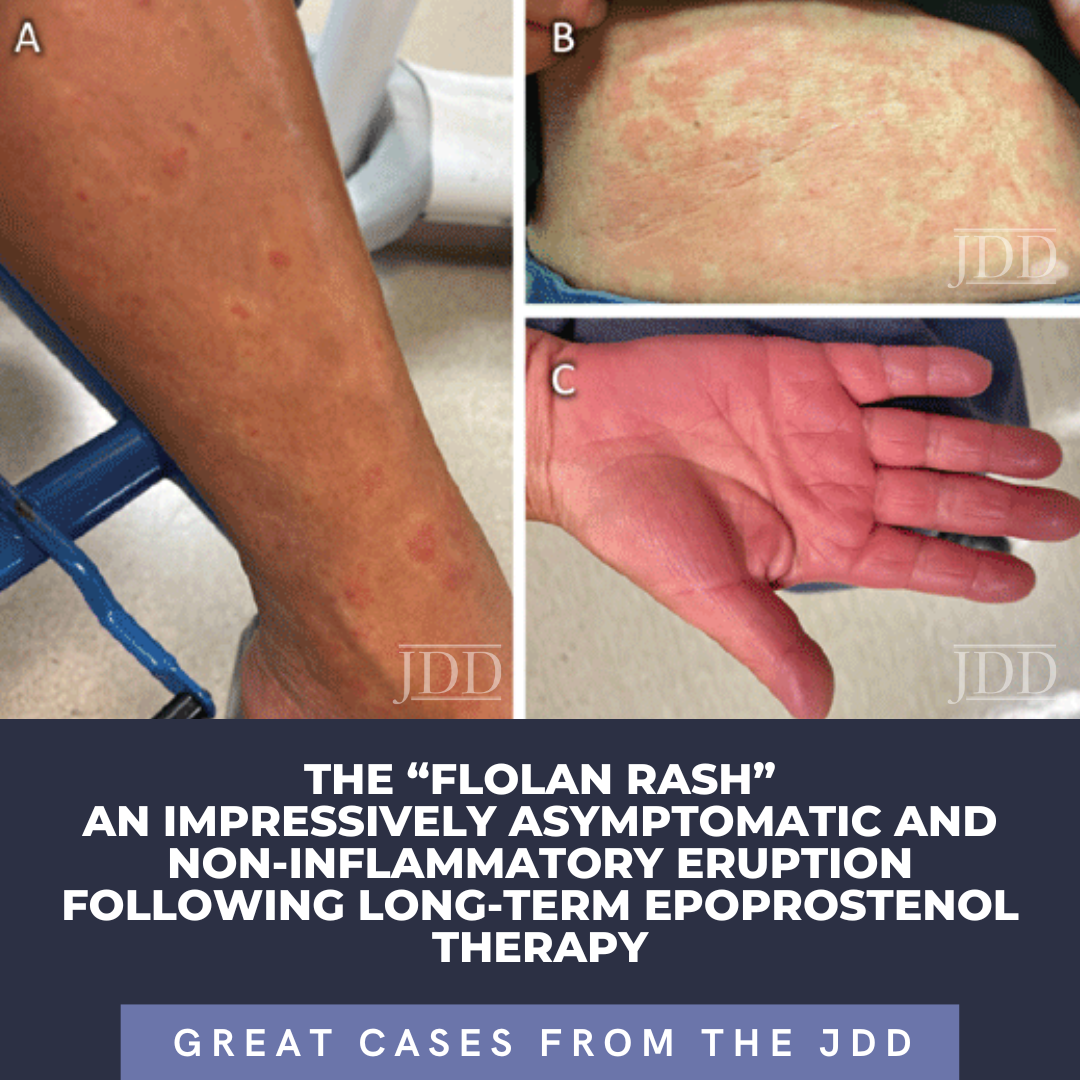
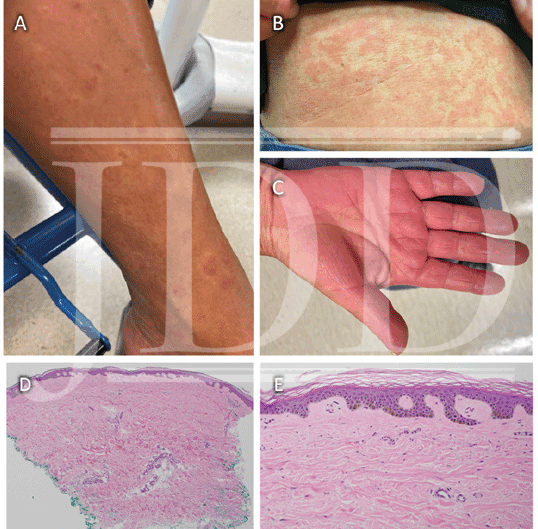
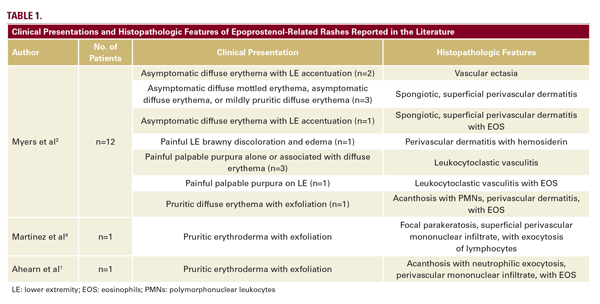
A case series by Myers et al, described long-standing, persistent skin eruptions following epoprostenol, and suggested a spectrum of drug-induced skin changes.2 Many presented with flushing and generalized blanching erythema, most prominent on the lower extremities, which is where biopsies were obtained. Myers also described a variety of histopathologic findings, including vascular ectasia interpreted to be stasis change, spongiotic dermatitis, and leukocytoclastic vasculitis (LCV) in a few patients with clinically purpuric lesions (Table 1).2 Similar to our patient, most were asymptomatic. In our case, the bright red eruption was thought primarily due to prostanoid vasodilatory effects, consistent with patients from the case series that presented with diffuse erythema and exhibited vascular ectasia. Noteworthy in our patient was a hypopigmented zone surrounding areas of erythema (Figure 1A). As epoprostenol is a prostacyclin analog, the mechanism of this halo may be similar to the Woronoff ring evident in psoriasis, which is due to local inhibition of prostaglandin E2 synthesis, leading to an inability to flush.6
While the incidence of PAH is low, dermatologists are likely to encounter patients on epoprostenol during their practice. We aim to increase awareness of the so-called “Flolan rash”, a persistent erythematous eruption that can commonly occur with long-term therapy.
DISCLOSURES
REFERENCES
2. Myers SA, Ahearn GS, Angelica Selim M, Tapson VF. Cutaneous findings in patients with pulmonary arterial hypertension receiving long-term epoprostenol therapy. J Am Acad Dermatol. 2004;51(1):98-102. doi:10.1016/j. jaad.2003.12.032
3. Fuentes A, Coralic A and Dawson KL. A new epoprostenol formulation for the treatment of pulmonary arterial hypertension. Am J Health Syst Pharm. 2012;69(16):1389-1393. doi:10.2146/ajhp110687
4. MartÃnez-MartÃnez ML, Escario-Travesedo E, Nuñez-Ares A, et al. Epoprostenol: Flushing or Erythroderma? Ann Dermatol. 2013;25(2):271- 272. doi:10.5021/ad.2013.25.2.271
5. Ricciotti E and FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986-1000. doi:10.1161/ATVBAHA.110.207449
6. Penneys NS, Ziboh V, Simon P, Lord J. Pathogenesis of Woronoff ring in psoriasis. Arch Dermatol. 1976;112(7):955-957.
SOURCE