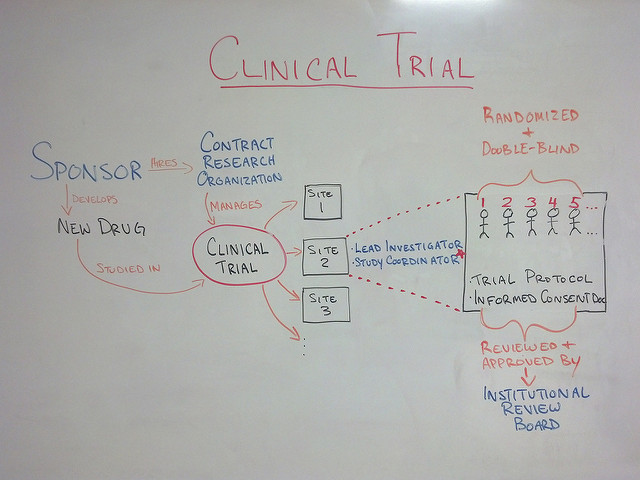
The practice of dermatology offers many avenues to expand one’s professional horizons and skills: surgical and/or cosmetic specialization, dermatopathology and pediatric dermatology, to name a few. One such area of sub-specialization is clinical research, namely pharmaceutical company-sponsored clinical trials. This is the process whereby new medications are tested prior to approval by the FDA, or the method by which existing products are approved for an additional indication. Breaking into the group of practices/physicians that perform clinical trials can be difficult, but not impossible. Let’s review some of the steps that a willing and interested physician can take to get involved with clinical trials.
1. Show an interest
Let representatives from the pharmaceutical industry know about your desire and ability to become a clinical investigator. This can start with your local sales representative or with a Medical Science Liaison (MSL). If you do not know the MSL, ask your sales representative to make an introduction. Some companies have MSLs and some do not.
2. Excel clinically in a specific disease area
It is easier to break into clinical trials if you have a particular area of clinical expertise, for example, acne or psoriasis. This makes you a “thought leader” or “key opinion leader” for that disease state. Pharmaceutical companies will naturally be more interested in your opinion and your ability to recruit patients for their studies if you have a larger group of patients from which to draw potential research subjects. Once you perform well in your area of expertise, you will be prepared to take on more studies in other areas.
3. Hone your skills of observation
Clinical research is not identical to clinical practice. It requires close observation of subtle changes in the disease state and/or the skin surrounding the lesions of that disease state. As a clinical investigator, one not only needs to appreciate differences in the overall disease state but minor details of the signs and symptoms of that disease state. In clinical practice, no one grades the scaling. Erythema and plaque elevation of psoriasis separately, but that is an absolute must in clinical trials.
4. Prepare yourself and your practice
One cannot simply start performing clinical trials without preparing oneself or one’s practice. Familiarize yourself with requirements and train one of your nurses or assistants to be a Research Coordinator. Performing clinical trials requires knowledge of Federal Codes and Regulations. Examples include Good Clinical Practices (GCPs), areas of locked cabinets for drug storage, temperature regulation and monitoring, and space for specialized equipment. There will be initial an investment of time and capital that will only pay off with proper preparation.
5. Perform
When one is selected to participate in a clinical trial, they should plan on recruiting effectively. This not only means getting the right number of patients in a timely fashion but getting quality patients and providing quality data is critical to one’s success and obtaining future trials.
6. Be innovative
Always think about and evaluate the product under study. Is there any additional information that the company should obtain? Is there something about the product that would make it suitable for another disease state? Would you be willing to perform an Investigator Initiated Trial on this or another product for this company? These are all questions that you can be asking yourself and the company during the course of a study. This will increase your value as an investigator.
If all of the above still seems interesting and not daunting, then clinical trials might be a good fit for you and/or your practice. Go for it and best of luck!
Did you enjoy this post? Find other articles on Becoming a Thought Leader by clicking here.
Featured image: Creative Commons whiteboard drawing showing a high-level view of what a Clinical Trial is by Jeremy Williams, licensed under CC BY 2.0