Pincer Nail Deformity in an African American Patient Corrected by Fractionated Carbon Dioxide Laser
 Introduction
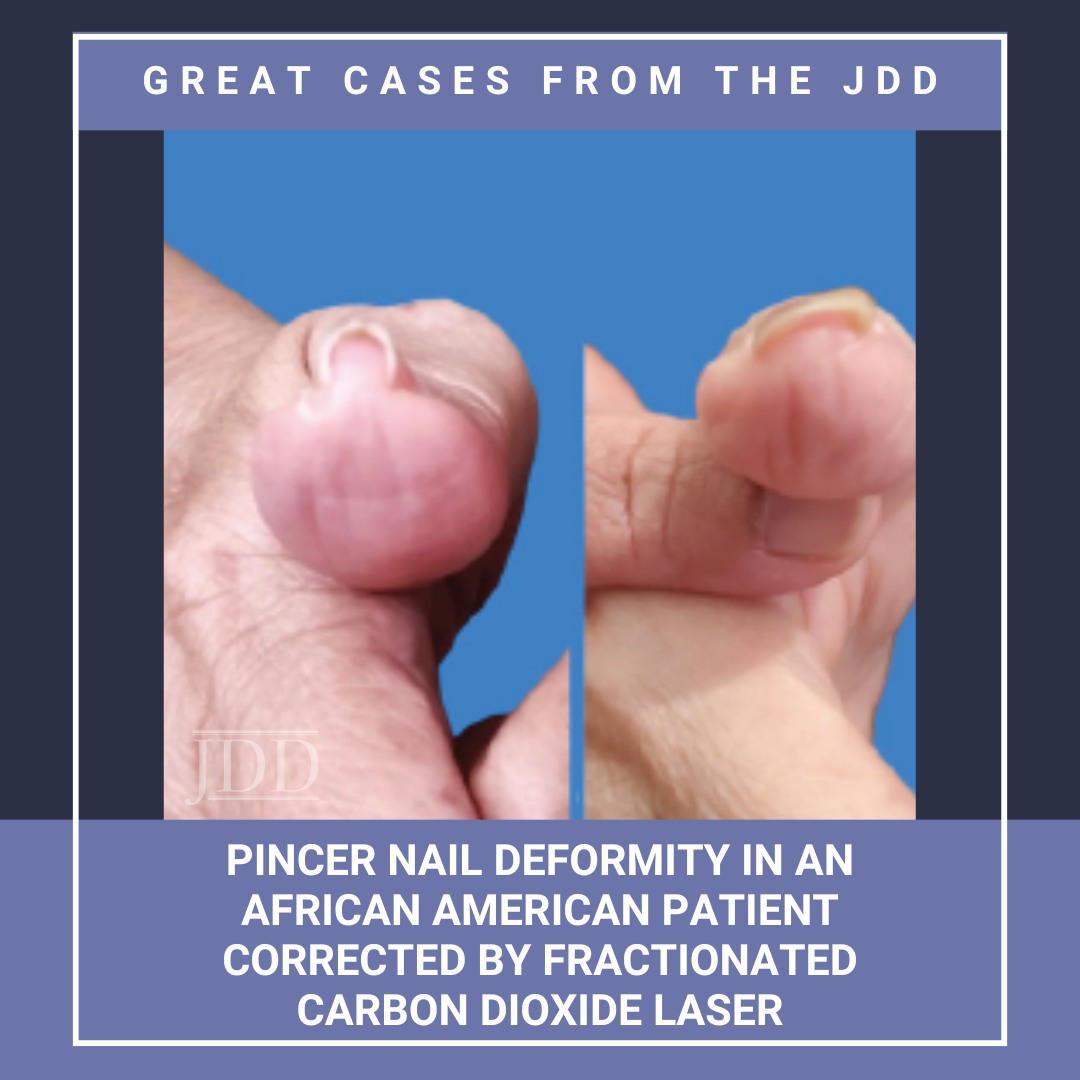
Pincer nail deformity is a painful nail condition characterized by excessive transverse curvature of the nail plate that pinches the surrounding tissue.1 This condition primarily involves the toenail of the hallux and is more frequently observed in older individuals.1 Management of a pincer nail typically involves nail braces or surgical removal.1 These managements usually fall sh …
Introduction
Pincer nail deformity is a painful nail condition characterized by excessive transverse curvature of the nail plate that pinches the surrounding tissue.1 This condition primarily involves the toenail of the hallux and is more frequently observed in older individuals.1 Management of a pincer nail typically involves nail braces or surgical removal.1 These managements usually fall sh …
 Introduction
Pincer nail deformity is a painful nail condition characterized by excessive transverse curvature of the nail plate that pinches the surrounding tissue.1 This condition primarily involves the toenail of the hallux and is more frequently observed in older individuals.1 Management of a pincer nail typically involves nail braces or surgical removal.1 These managements usually fall sh …
Introduction
Pincer nail deformity is a painful nail condition characterized by excessive transverse curvature of the nail plate that pinches the surrounding tissue.1 This condition primarily involves the toenail of the hallux and is more frequently observed in older individuals.1 Management of a pincer nail typically involves nail braces or surgical removal.1 These managements usually fall sh … 

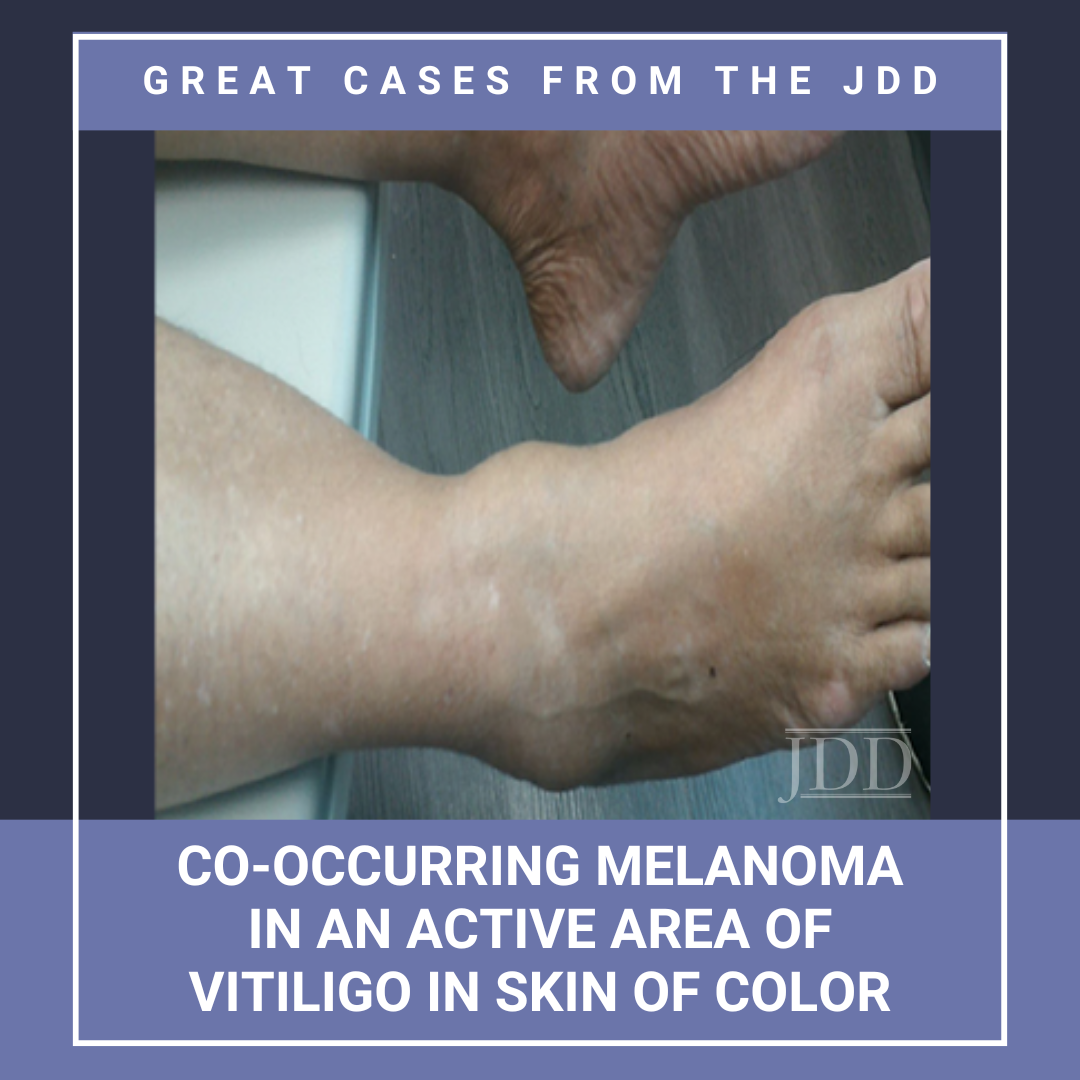 The association between vitiligo and melanoma is complex. While the incidence of vitiligo in patients with melanoma is higher, the risk for the reverse, ie, the development of melanoma in a patient with vitiligo, is thought to be decreased. This report presents a case of melanoma developing on a non-sun-exposed site in a patient with skin of color and untreated vitiligo. It emphasizes the need …
The association between vitiligo and melanoma is complex. While the incidence of vitiligo in patients with melanoma is higher, the risk for the reverse, ie, the development of melanoma in a patient with vitiligo, is thought to be decreased. This report presents a case of melanoma developing on a non-sun-exposed site in a patient with skin of color and untreated vitiligo. It emphasizes the need … 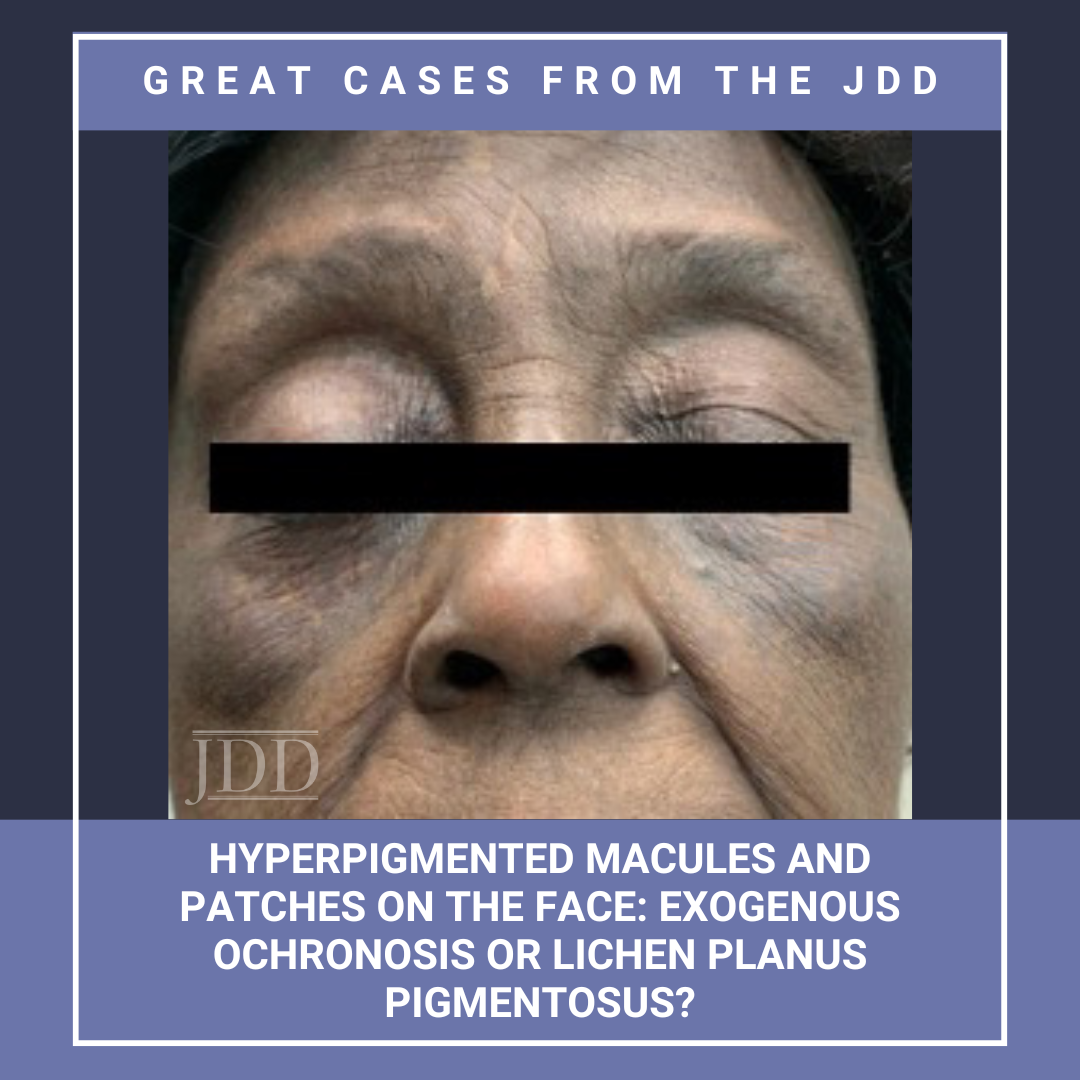 JDD authors Deepika Narayanan, MD and Stephen K. Tyring, MD, PhD, MBA present a case of a patient with a 10-year history of blue-black macules and patches on the face and an associated history of skin- lightening cream usage. The skin lightening cream contained hydroquinone, which is often associated with exogenous ochronosis (EO). Interestingly, the biopsy did not show characteristic findings of …
JDD authors Deepika Narayanan, MD and Stephen K. Tyring, MD, PhD, MBA present a case of a patient with a 10-year history of blue-black macules and patches on the face and an associated history of skin- lightening cream usage. The skin lightening cream contained hydroquinone, which is often associated with exogenous ochronosis (EO). Interestingly, the biopsy did not show characteristic findings of … 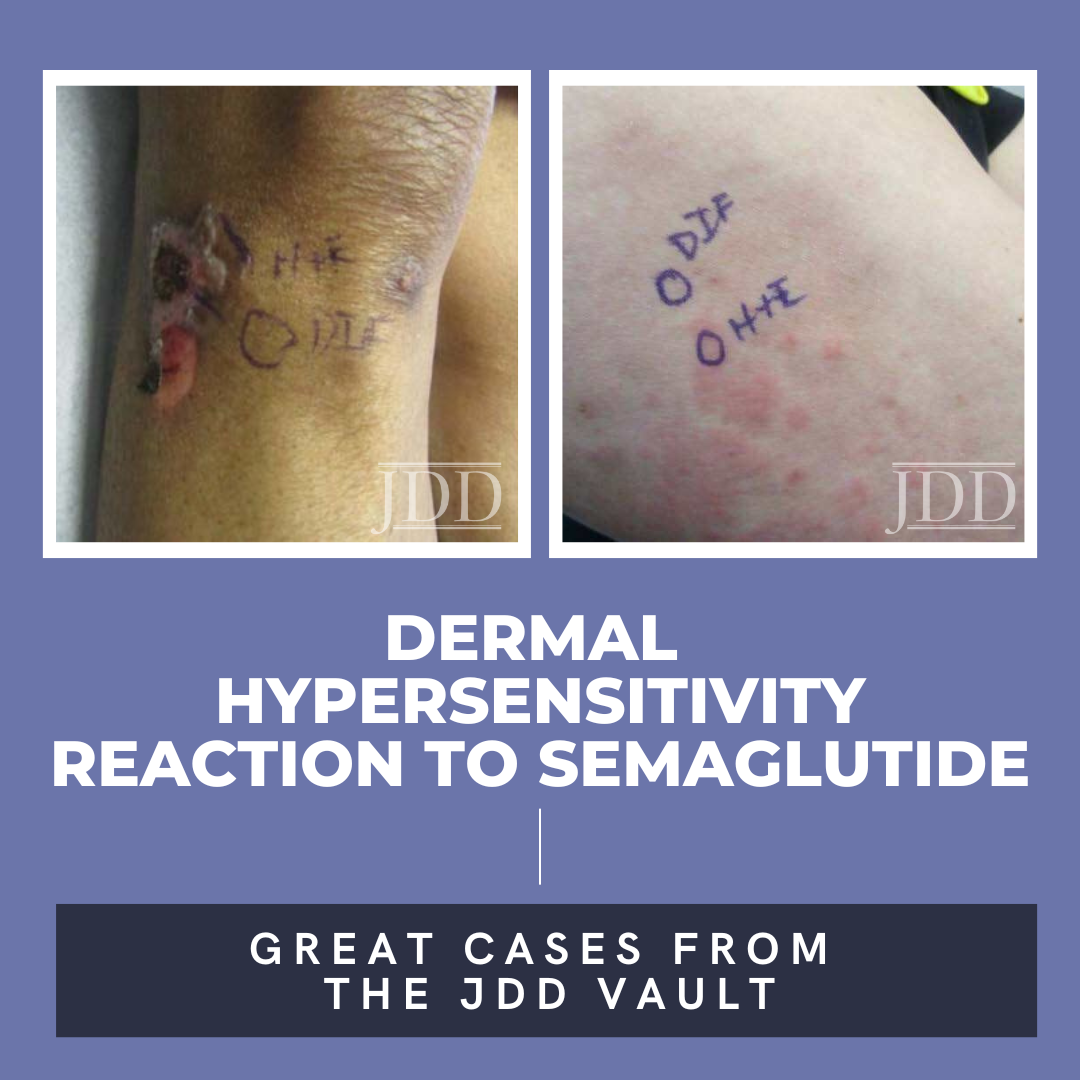 Semaglutide is a glucagon-like peptide-1 (GLP-1) analog that was FDA-approved in 2017 for treatment of type II diabetes and in 2021 for treatment for chronic weight management in adults with obesity or overweight with at least one weight-related condition.1 Due to its longer duration of action, it is typically administered subcutaneously once weekly. The safety profile of semaglutide is similar to …
Semaglutide is a glucagon-like peptide-1 (GLP-1) analog that was FDA-approved in 2017 for treatment of type II diabetes and in 2021 for treatment for chronic weight management in adults with obesity or overweight with at least one weight-related condition.1 Due to its longer duration of action, it is typically administered subcutaneously once weekly. The safety profile of semaglutide is similar to … 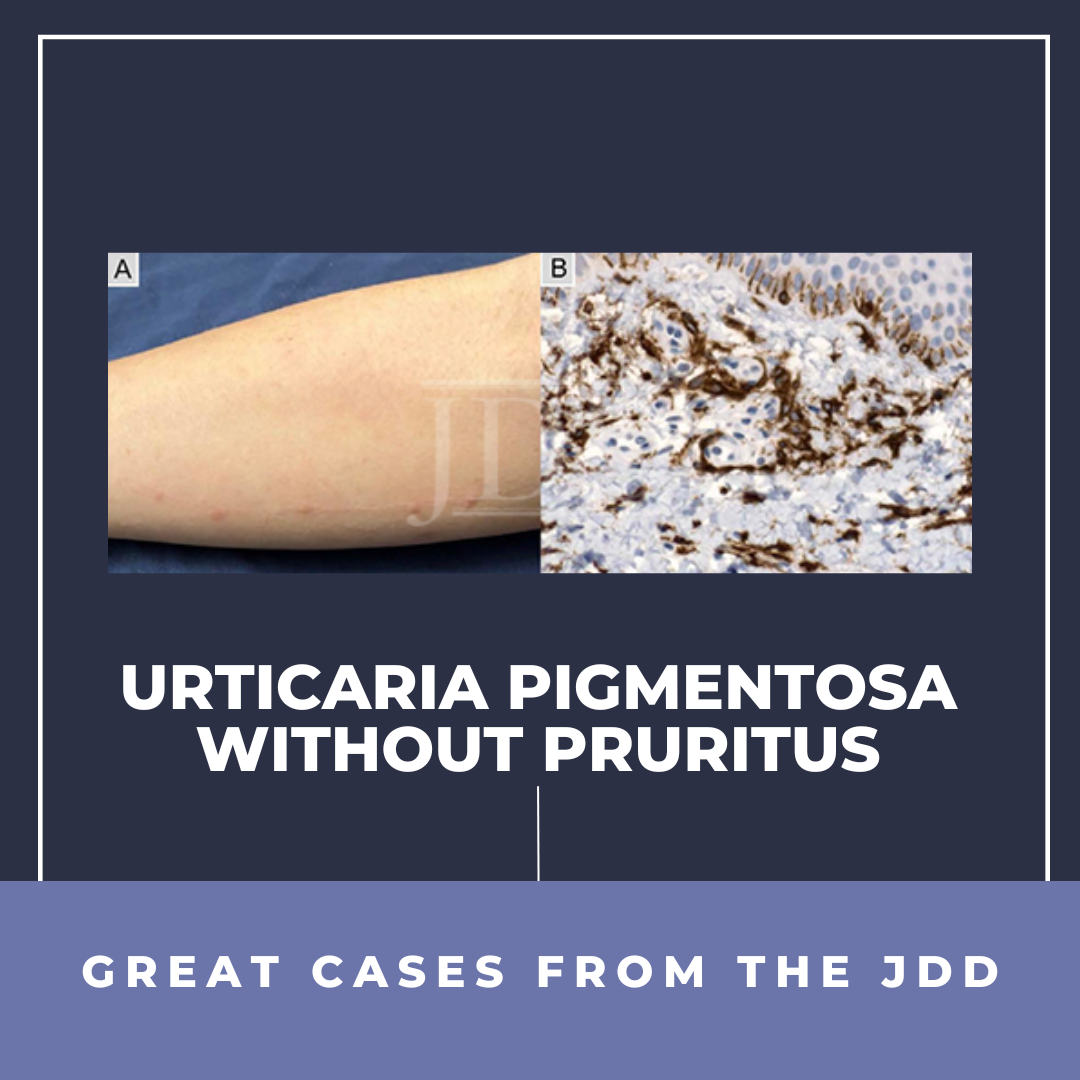 Mastocytosis is a group of disorders characterized by the pathologic accumulation of mast cells in various tissues. One example of mastocytosis is urticaria pigmentosa, which presents with mastocytomas that can cause hives and, when irritated, pruritus. To the authors' knowledge, they are describing the first case of urticaria pigmentosa without pruritus. The patient had a positive Darier' …
Mastocytosis is a group of disorders characterized by the pathologic accumulation of mast cells in various tissues. One example of mastocytosis is urticaria pigmentosa, which presents with mastocytomas that can cause hives and, when irritated, pruritus. To the authors' knowledge, they are describing the first case of urticaria pigmentosa without pruritus. The patient had a positive Darier' …