Dermal Hypersensitivity Reaction to Semaglutide: Two Case Reports
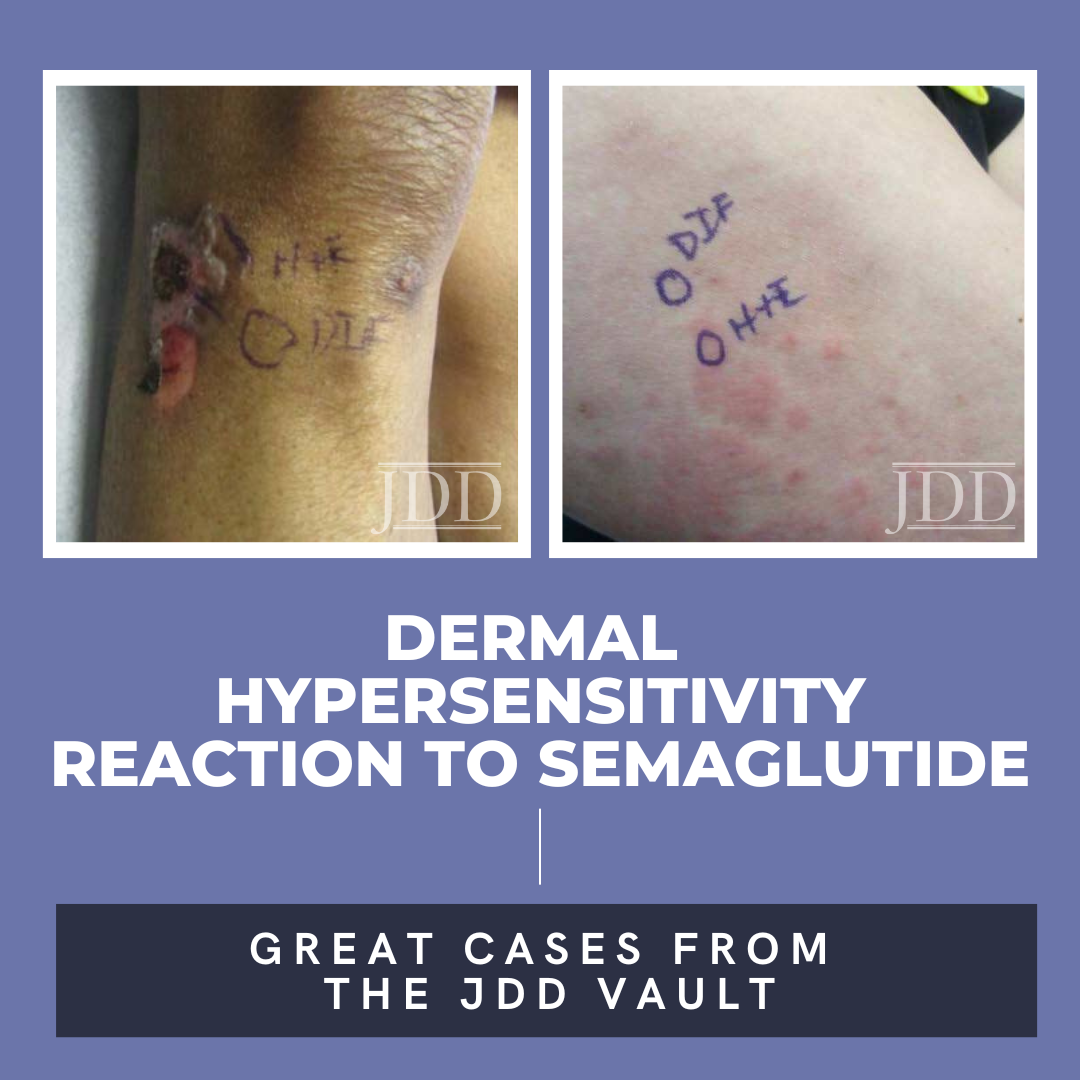 Semaglutide is a glucagon-like peptide-1 (GLP-1) analog that was FDA-approved in 2017 for treatment of type II diabetes and in 2021 for treatment for chronic weight management in adults with obesity or overweight with at least one weight-related condition.1 Due to its longer duration of action, it is typically administered subcutaneously once weekly. The safety profile of semaglutide is similar to …
Semaglutide is a glucagon-like peptide-1 (GLP-1) analog that was FDA-approved in 2017 for treatment of type II diabetes and in 2021 for treatment for chronic weight management in adults with obesity or overweight with at least one weight-related condition.1 Due to its longer duration of action, it is typically administered subcutaneously once weekly. The safety profile of semaglutide is similar to …
 Semaglutide is a glucagon-like peptide-1 (GLP-1) analog that was FDA-approved in 2017 for treatment of type II diabetes and in 2021 for treatment for chronic weight management in adults with obesity or overweight with at least one weight-related condition.1 Due to its longer duration of action, it is typically administered subcutaneously once weekly. The safety profile of semaglutide is similar to …
Semaglutide is a glucagon-like peptide-1 (GLP-1) analog that was FDA-approved in 2017 for treatment of type II diabetes and in 2021 for treatment for chronic weight management in adults with obesity or overweight with at least one weight-related condition.1 Due to its longer duration of action, it is typically administered subcutaneously once weekly. The safety profile of semaglutide is similar to … Continue reading "Dermal Hypersensitivity Reaction to Semaglutide: Two Case Reports"


 The Journal of Drugs in Dermatology welcomes 2024 with a strong line up of original articles, case reports, brief communications, and letters to the editor. Straight from the Editor's desk, we share this month's issue highlights!
ORIGINAL ARTICLES
Current Landscape of Hyaluronic Acid Filler Use in the United States shares the results of a comprehensive search of all the FDA approved dermal fill …
The Journal of Drugs in Dermatology welcomes 2024 with a strong line up of original articles, case reports, brief communications, and letters to the editor. Straight from the Editor's desk, we share this month's issue highlights!
ORIGINAL ARTICLES
Current Landscape of Hyaluronic Acid Filler Use in the United States shares the results of a comprehensive search of all the FDA approved dermal fill …