INTRODUCTION
Alopecia universalis is a type of alopecia areata (AA) characterized by total body hair loss. The mechanism of this loss is primarily due to autoimmune disruption of the hair follicle. AA has historically been treated with corticosteroids, minoxidil, and other immune therapies that possess limited efficacy with high recurrence rates and adverse effects.1 However, in recent years the Janus Kinase inhibitors (JAKis) have emerged with novel immunomodulatory effects by blocking interferon-gamma and interleukin-15, involved in immune activation at the hair follicle.2 Currently, three oral JAKis, baricitinib, ritlecitinib, and deuruxolitinib, have been studied under phase 3, randomized, controlled trials for AA.2-5
CASE REPORT/CASE PRESENTATION
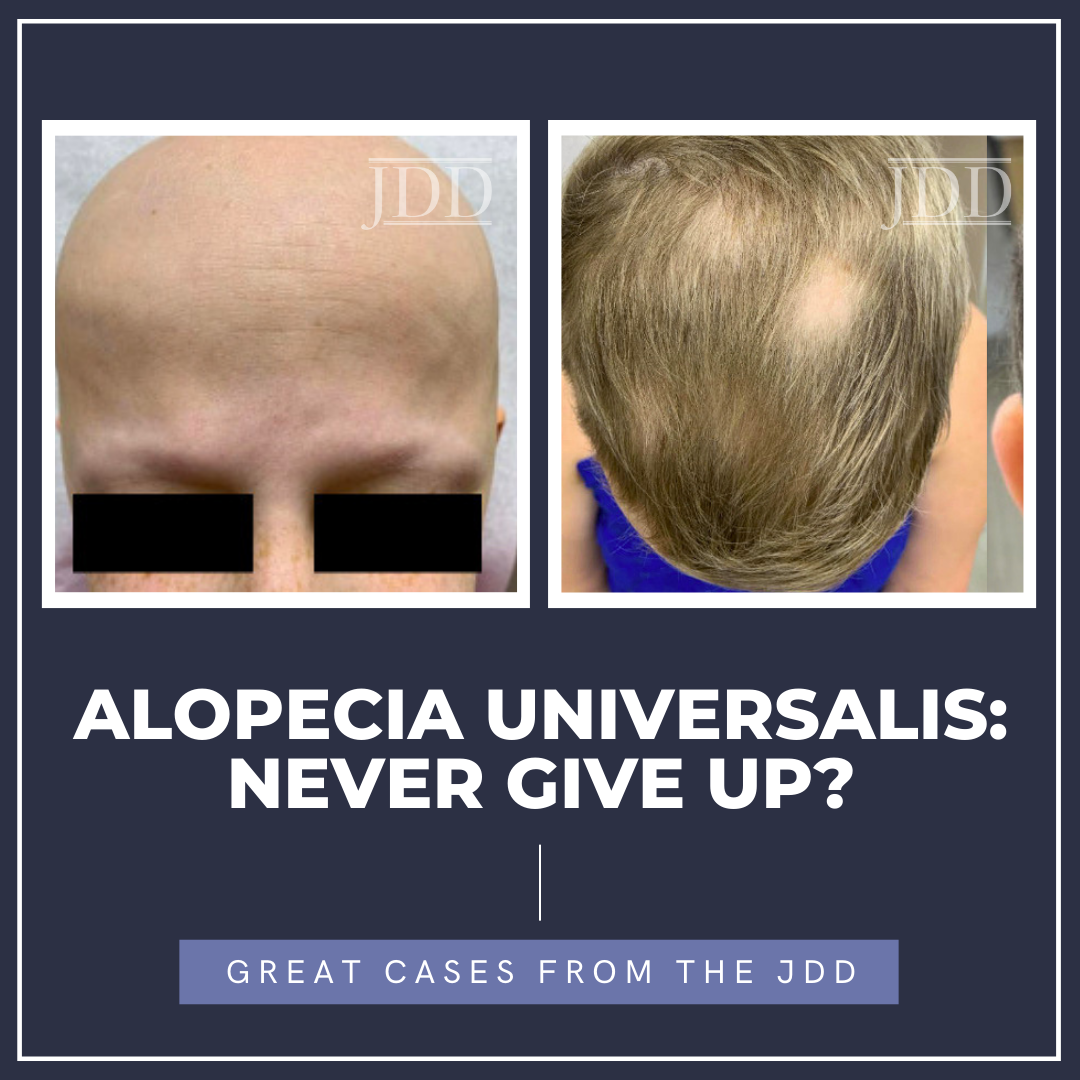
We present the case of a 28-year-old female with Alopecia Universalis who experienced rapid onset improvement in hair growth over a 6-month period after 18 total months of JAKi therapy. The patient initially presented to our institution’s dermatology clinic with abrupt onset loss of scalp hair with progression of total body hair loss within a few months. At that time, her exam showed scattered terminal hairs on the scalp with few vellus hairs as well as nail pits on multiple nails. The patient’s past medical history was significant for Turner’s syndrome, hypothyroidism, atopic dermatitis, and an isolated episode of unspecified alopecia at age 11. Her mother reportedly also has a history of alopecia areata. The patient was initially started on an oral prednisone taper, topical corticosteroids, and minoxidil titrated up to 3.75mg with poor response. After 3 months with little to no improvement, baricitinib was added at 2mg and was promptly titrated to 4mg.
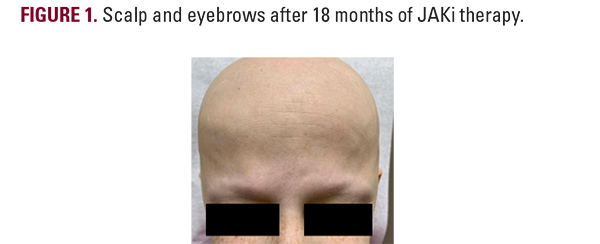
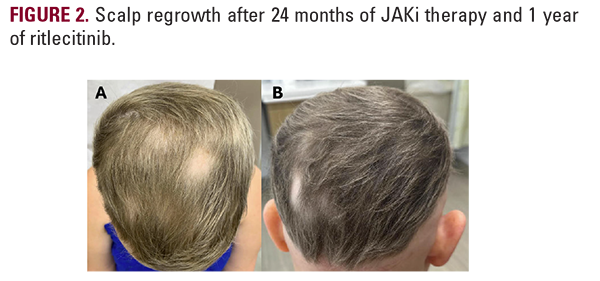
Over the next 6 months, the patient noted no regrowth of hair on any body surface area and down-titrated baricitinib to 2mg due to difficulty tolerating the higher dose and minoxidil to 2.5mg, due to weight gain. After one full year on baricitinib, the patient’s Severity of Alopecia Tool (SALT) remained at 100%, but she did note improvement in her nail pitting. Her therapy was switched to ritlecitinib at 50mg adjunct with 1.25mg minoxidil, and at 6-month follow up, she began to notice some regrowth of eyelashes and eyebrows as well as the return of fine, vellus hair on the scalp, as seen in Figure 1. With the promising return of vellus hair, the same regimen was continued and after approximately one year of ritlecitinib and two total years of JAKi therapy, she had remarkable improvement in regrowth of terminal hairs on the vertex scalp, eyelashes, and eyebrows, as seen in Figure 2, with a SALT score of 17.2. The patient did not yet have axillary or body hair regrowth. Isolated patches of scalp hair loss were treated with intralesional triamcinolone acetonide. Overall, the patient tolerated both JAK inhibitors well throughout treatments without adverse effects.
DISCUSSION
Prior to the emergence of JAK inhibitors, alopecia areata, particularly universalis, was known to be highly refractory to therapy. For severe and recalcitrant disease, this drug class has proven its efficacy in clinical trials, and baricitinib became the first-in-disease FDA-approved treatment in 2022.6 Trial outcomes were assessed via primary endpoints of SALT scores <20 at 36 and 24 weeks for baricitinib and ritlecitinib, respectively, in comparison to placebo.3-4 In this trial, atopic history and the presence of alopecia universalis were reported, but no data was reported regarding the association of these factors with primary endpoints. In the ritlecitinib trial, higher baseline SALT scores of individuals with alopecia totalis and alopecia universalis were reported, but subgroup analyses demonstrated consistency of treatment effect compared to those without alopecia totalis or alopecia universalis.4 Post-hoc analyses have also supported ritlecitinib efficacy independent of hair loss profile at baseline.7
Our patient had an initial SALT score of 100 and failed to improve at 1 year of treatment with baricitinib, and it was not until 6 months of ritlecitinib therapy, she began to see vellus hair regrowth. Of note, improvement to a SALT score of <20 was necessary to meet primary endpoints in both baricitinib and ritlecitinib clinical trials, which this patient would not have met until more than 1.5 years of total JAKi treatment. It is also notable that trial patients were excluded based on the use of or failure of any previous JAKi therapy.3,4 This patient’s case poses the question of whether their hair re-growth was responsive to prolonged JAKi therapy in general or if the trial of ritlecitinib was more efficacious than baricitinib. The presence of baseline nail pitting in this patient has also been considered to reflect AA disease severity.8 Nail pitting resolution in this patient preceded hair regrowth by many months and may be a positive prognosticator in eventual hair regrowth. Additionally, AA has been suggested to be more prevalent in Turner’s syndrome, along with other autoimmune conditions, such as hypothyroidism, as seen in this patient.9 Important considerations in this case include the unique factors that may have contributed to this patient being an initial poor responder to JAKi including atopic history, Turner’s syndrome, and presence of nail pitting. The novelty and significance of this case lie in the prolonged latency period between JAKi initiation and treatment response. Providers should be fervent in the treatment of recalcitrant alopecia areata by providing adequate trials of at least 1 year with each individual therapy if possible. This case lends insight into the safety of JAKis’ long-term use, as evidenced by the lack of adverse effects for this patient. Further trials and reports outside of trial settings are needed for this patient population to compare the efficacies of JAKis to each other and in the long term.
CONCLUSION
The authors aim to highlight a real-world case of delayed JAKi response in a 28-year-old patient with alopecia universalis refractory to baricitinib with an eventual robust response to ritlecitinib. In this case, JAKis were well tolerated for a prolonged course. After 2 years of total treatment, this patient’s outcomes demonstrate the benefit of diligence when trialing JAKis as well as attempting alternative medications within the class when treatment response is inadequate.
REFERENCES
-
- Zhou C, Li X, Wang C, Zhang J. Alopecia areata: an update on etiopathogene-sis, diagnosis, and management. Clin Rev Allergy Immunol. 2021;61(3):403-423. doi:10.1007/s12016-021-08883-0
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89(2S):S29-S32. doi:10.1016/j.jaad.2023.05.049
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alope-cia areata. N Engl J Med. 2022;386(18):1687-1699. doi:10.1056/NEJMoa2110343
- King B, Zhang X, Harcha WG, et al. Efficacy and safety of ritlecitinib in adults and adolescents with alopecia areata: a randomised, double-blind, mul-ticentre, phase 2b-3 trial [published correction appears in Lancet. 2023; 401(10392):1928. doi: 10.1016/S0140-6736(23)01078-4]. Lancet. 2023;401 (10387):1518-1529. doi:10.1016/S0140-6736(23)00222-2
- King B, Mesinkovska N, Mirmirani P, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus Kinase inhibitor, in moderate-to-severe al-opecia areata. J Am Acad Dermatol. 2022;87(2):306-313. doi:10.1016/j.jaad.2022.03.045
- Lensing M, Jabbari A. An overview of JAK/STAT pathways and JAK inhibiti-on in alopecia areata. Front Immunol. 2022;13:955035. doi:10.3389/fimmu.2022.955035
- Thaçi D, Tziotzios C, Ito T, et al. Hair loss profiles and ritlecitinib efficacy in patients with alopecia areata: post hoc analysis of the allegro phase 2b/3 stu-dy. Dermatol Ther (Heidelb). 2023;13(11):2621-2634. doi:10.1007/ s13555-023-00997-x
- Roest YBM, van Middendorp HT, Evers AWM, et al. Nail involvement in al-opecia areata: a questionnaire-based survey on clinical signs, impact on quality of life and review of the literature. Acta Derm Venereol. 2018;98(2):212-217. doi:10.2340/00015555-2810
SOURCE
Jiminez, Victoria, et al. “Alopecia Universalis: Never Give Up?.” Journal of drugs in dermatology: JDD 24.3 (2025): 316-318.
Content and images used with permission from the Journal of Drugs in Dermatology.
Adapted from original article for length and style.
Did you enjoy this JDD case report? You can find more here.