On this Mnemonic Monday, we challenge you to remember syndromes with an X-linked Recessive (XLR) inheritance pattern with the following mnemonic:
CHAD’S KINKY WIFE GOT LUCKY
Chronic granulomatous disease
Hunter’s disease
Anhidrotic ectodermal dysplasia
Dyskeratosis congenita
SCID
Menkes Kinky hair disease
Wiskott-Aldrich
Ichthyosis
X-linked Fabry’s disease
Ehlers-Danlos V and IX
G6PD deficiency
Lesch-Nyhan
Click HERE to print your mnemonic card.
Study More!
Need a refresher on X-Linked Recessive (XLD) Syndromes? Check out the following pages of your 2019 Derm In-Review Study Guide:
X-Linked Recessive Syndromes: 210
Don’t have a copy? Sign up for Derm In-Review and download the digital version here.
Further Reading
Check out the following case report published in the Journal of Drugs in Dermatology (JDD):
X-linked Chronic Granulomatous Disease With Voriconazole-induced Photosensitivity/ Photoaging Reaction
Abstract
Due to the mutations in the nicotinamide dinucleotide phosphate (NADPH) oxidase complex in X-linked chronic granulomatous disease (CGD), the phagocytic activity in these patients is curtailed by a dysfunctional respiratory burst. This can lead to life-threatening bacterial and fungal infections. The prognosis for patients with CGD has dramatically improved with the advent of effective prophylactic drugs targeting catalase-positive bacteria, opportunistic Gram-negative bacteria, and fungi. Voriconazole, a second-generation triazole, is a commonly used agent for prophylaxis in this population. The authors report a case of photoaging and photosensitivity reaction associated with voriconazole exposure in a 10-year-old boy with X-linked CGD. With broad-spectrum sunscreen, topical steroids, and discontinuation of voriconazole, the patient showed significant improvement.
Introduction
Chronic granulomatous disease (CGD) is an X-linked or autosomal recessive disorder characterized by a mutation in the nicotinamide dinucleotide phosphate (NADPH) oxidase complex. People afflicted with this disorder are unable to generate microbicidal reactive products such as superoxide anion, hydrogen peroxide, hydroxyl anion, and hypohalous acid within their phagocytes. As a result of the impaired phagocytic activity, CGD patients suffer from recurrent life-threatening bacterial and fungal infections. Most infections reported are caused by a specific subset of pathogens: Staphylococcus aureus (S. aureus), Burkholderia cepacia (B. cepacia), Serratia marcescens (S. marcescens), Nocardia, and Aspergillus species such as Aspergillus fumigatus (A. fumigatus) and Aspergillus nidulans (A. nidulans).
Currently, treatment of CGD includes prophylactic antimicrobials targeting catalase-positive bacteria such as Staphylococcus species, opportunistic Gram-negative bacteria, and fungi. Daily trimethoprim-sulfamethoxazole (TMP-SMZ) and itraconazole or voriconazole are often used.¹ In addition, interferon-gamma therapy has been found to augment oxidant-independent antimicrobial pathways. One to two percent of the general population treated with voriconazole experience dermatologic side effects which include various types of dermatologic conditions such as cheilitis, facial erythema, discoid lupus erythematosus, Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme, and photosensitivity reactions.² The incidence of such reactions in CGD patients is unknown. The authors present a case of voriconazole-induced photosensitivity and photoaging in a 10-year-old boy with chronic granulomatous disease.
Case Report
A 10-year-old white male presented with a three-week history of a pruritic, photodistributed rash over the face, arms, and lower legs. He had a history of X-linked CGD diagnosed by nitroblue tetrazolium assay at four months of age. Shortly after birth, he had had recurrent fevers, Serratia infections, and pneumonia. He had been taking prophylactic TMP-SMZ and interferon gamma-1b since four months of age and voriconazole for the past three years. Upon further questioning, it was discovered that nine weeks prior to his presentation, his voriconazole dose had been doubled from 200–400 mg by mouth every day due to fever of unknown origin, which resolved after the increased dose. He also admitted to a recent increase in sun exposure.
On physical exam, he was noted to be short in stature. His face and neck demonstrated diffuse erythema with multiple, scattered lentigines (Figure 1). His arms and legs also demonstrated sharply demarcated pink, scaly plaques with multiple lentigines in a photo distributed pattern (Figures 2a and 2b). Based on the patient’s physical findings, his recent increase in voriconazole, and an increase in sun exposure, the authors diagnosed the patient with voriconazole-induced photosensitivity and photoaging reaction. He was started on broad-spectrum sunscreen and topical steroids and showed significant improvement within two weeks. He was also referred to his immunologist who switched him from voriconazole to itraconazole prophylaxis.
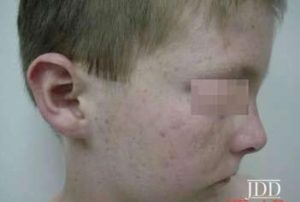
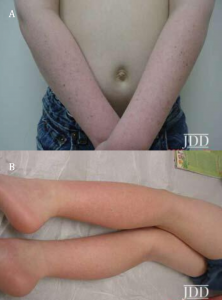
pattern on arms and legs
Discussion
Voriconazole is a second-generation triazole with broad-spectrum coverage of clinically significant fungal infections caused by Aspergillus, Scedosporium apiospermum (S. apiospermum), Fusarium, and Candida. It is fungicidal by inhibiting 14-alphasterol demethylase, which is necessary for ergosterol biosynthesis. It is both a substrate and an inhibitor of the hepatic P450 isoenzyme pathways CYP2C19, CYP2C9, and CYP3A4, but is primarily cleared by CYP2C19. Three to five percent of Caucasians and African Americans possess a genetic polymorphism for CYP2C19 and are thus poor metabolizers of voriconazole who will achieve a four-fold higher voriconazole concentration. For children under the age of 12, it has been found that voriconazole at standard doses is cleared more rapidly and thus requires higher doses to achieve therapeutic plasma levels than in adults. The most common side effects of voriconazole therapy are visual, hepatic, and dermatologic abnormalities. Transient visual disturbances such as altered or enhanced visual perception, blurred vision, color vision change, and photophobia have been reported. Liver function tests should be followed as elevation in transaminases has been noted and rarely hepatitis and fulminant hepatic failure have been seen. Mild skin rashes and photosensitivity have been reported, especially during long-term treatment. More severe skin reactions, such as erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis, have also been reported.³
The cause of voriconazole-induced photosensitivity is unknown, but there are a number of theories present in the literature. The retinoid model suggests that voriconazole directly inhibits P450 isoenzymes, leading to elevated levels of all-trans-retinol and 13-cis-retinol. This theory is supported by the observation that patients who received voriconazole subsequently developed facial erythema and cheilitis and were noted to have an upper limit of normal all-trans-retinal plasma levels.² While it is more likely that the phototoxic and photoaging effects are side effects solely attributable to voriconazole exposure, one might also consider the effects of voriconazole and trimethoprim-sulfamethoxazole when given in combination since daily trimethoprim-sulfamethoxazole (5 mg/kg/day) for bacterial prophylaxis is a central component of CGD management.5
Trimethoprim is mostly excreted unchanged in the urine, although 20% is metabolized by cytochrome P450 isoforms. Sulfamethoxazole is mainly metabolized by CYP2C9, a pathway that also metabolizes voriconazole. While previous in vitro studies have shown that sulfamethoxazole inhibits CYP2C9, there are no published in vivo studies that examine the effects of trimethoprim and sulfamethoxazole on different P450 isoforms. Combining voriconazole, which is metabolized by CYP2C19, CYP2C9, and CYP3A4, with trimethoprim-sulfamethoxazole, which has been shown to inhibit these isoenzymes, could lead to elevated voriconazole levels and thus exacerbate the photosensitivity. In vivo studies are needed to validate the findings of this in vitro study.3
There are a handful of case reports in the literature of voriconazole- induced photosensitivity reactions in CGD patients. Rubenstein et al.7 published two cases of voriconazole-induced photosensitivity in two pediatric patients with CGD. They reported a photo-induced drug eruption similar to our patient in an eleven-year-old boy who was taking 200 mg voriconazole twice a day for two weeks to treat chronic invasive aspergillosis. The drug was continued but the patient was instructed to avoid sun exposure and he did not develop any further photosensitivity reactions. In the second case, a 13-year-old boy developed a more severe reaction of photo distributed erythema of his face, dorsal hands, and feet with desquamation of lips, palms, and soles after two weeks of voriconazole therapy. Voriconazole was discontinued and replaced with itraconazole at which time his symptoms resolved.7 Published studies have found that photosensitivity is more likely to develop when treated for 12 weeks or longer but tends to resolve upon discontinuation of voriconazole.
While photosensitivity reactions have been observed, photoaging effects are a much rarer occurrence. There are only two previous reports of photoaging in the literature. In 2005, Racette et al. published a case report of photoaging and phototoxicity due to long-term voriconazole treatment. A 15-year-old immunocompetent female patient was treated with voriconazole for 11 months for a fungal sinus infection which had invaded the cranium. After six to seven weeks on voriconazole therapy, she developed cheilitis, facial erythema, and numerous dark nevi on her scalp and chest. Five months later, she developed increasing erythema and scaling of the face, arms, and legs, with lentigines and ephelides on the back of her arms and legs. Subsequently, flaccid fragile blisters erupted on the anterior side of her lower legs. Racette et al.2 suggested a possible phototoxic effect of UK-121,265, the major metabolite of voriconazole. As this case report illustrates, the length of treatment appears to be an important variable.2
Perhaps the most striking case report of voriconazole-induced photoaging comes out of Australia. McCarthy et al. reported a 32-year-old woman with CGD who received long-term voriconazole therapy for recurrent multifocal aspergillosis. She was started on voriconazole 400 mg by mouth twice a day. Shortly after initiating voriconazole, she developed a severe photosensitivity reaction. She continued voriconazole therapy with emollient, anti-inflammatory, and sunscreen creams in addition to sun avoidance. Nevertheless, her photosensitivity reaction intensified over the years and she developed a squamous cell carcinoma on her upper lip three years later. She then developed multifocal, highly invasive squamous cell carcinomas on her nose and cheek within the next year. She was finally switched from voriconazole to posaconazole therapy and within 14 days the photosensitivity reaction completely resolved. The combined effects of long-term voriconazole treatment, high UV exposure, and immunocompromised state were all believed to have contributed to the development of her squamous cell carcinomas.9
Clinicians treating immunocompromised patients who require long-term voriconazole treatment should be aware of the risk of photosensitivity and photoaging. They should educate these patients about the risk of developing these reactions and urge them to avoid sun exposure and use sunscreen. Furthermore, in most case reports, the patients’ symptoms resolved when switched from voriconazole to another triazole such as itraconazole or posaconazole.
References
1. Segal BH, Leto, TL, Gallin, JI, et al. Genetic, biochemical and clinical features of chronic granulomatous disease. Re Molecul Med. 2000;79(3):170-200.
2. Racette AJ, Roenigk HH Jr, Hansen R, et al. Photoaging and phototoxicity from long-term voriconazole treatment in a 15-year-old girl. J Am Acad Dermatol. 2005;52(5 Suppl):S81-S85.
3. Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/Pharmacodynamic profile of voriconazole. Clin Pharmacokinet. 2006:45(7):649-663.
4. Holland SM. Chronic granulomatous disease. Clinic Rev Allerg Immunol. Published online: 09 June 2009.
5. Rubenstein M, Levy ML, Metry D. Voriconazole-induced retinoidlike photosensitivity in children. Pediatr Dermatol. 2004;21(6):675- 678.
6. McCarthy KL, Playford EG, Looke DF, Whitby M. Severe photosensitivity causing multifocal squamous cell carcinomas secondary to prolonged
Case Report content and images used with permission from the Journal of Drugs in Dermatology.
Test your knowledge!

A 31-year-old male with a history of recurrent febrile episodes presents with the skin findings in the above photos. The gene that is mutated in this condition encodes for what type of protein?
A: Tyrosine kinase receptor
B: Protein tyrosine-protein phosphatase
C: Telomerase complex
D: DNA helicase
E: Nuclear envelope protein
To find out the correct answer and read the explanation, click here.
Did you enjoy this mnemonic? You can find more here.
Brought to you by our brand partner Derm In-Review. A product of SanovaWorks.

