HMF has a clinically benign course and responds well to therapy; however, relapse is common.³ We report a case of HMF misdiagnosed as vitiligo in order to illuminate diagnostic, histopathological, and treatment modalities.
CASE REPORT
A 21-year-old woman of Fitzpatrick skin type V presents with a 6-month history of progressive, pruritic hypopigmented macules, without redness or scale, involving the upper and lower extremities. She describes sparing of the face and torso. She denies a preceding upper respiratory or gastrointestinal infection. She does not report associated constitutional symptoms including fevers, chills, or night sweats. She has no prior personal or family history of dermatitis, psoriasis, vitiligo, or autoimmunity. Prior diagnosis made by 4 local physicians included vitiligo.
On examination, she has Fitzpatrick skin type V. On the upper and lower extremities bilaterally, including the dorsal hands and feet, she has variably sized, 6mm to 1cm in diameter, hypopigmented macules with subtle pink erythema. Sparing of the face and torso is noted. No overlying scale present. Lesional and uninvolved skin biopsies were done from corresponding sites on her bilateral arms.
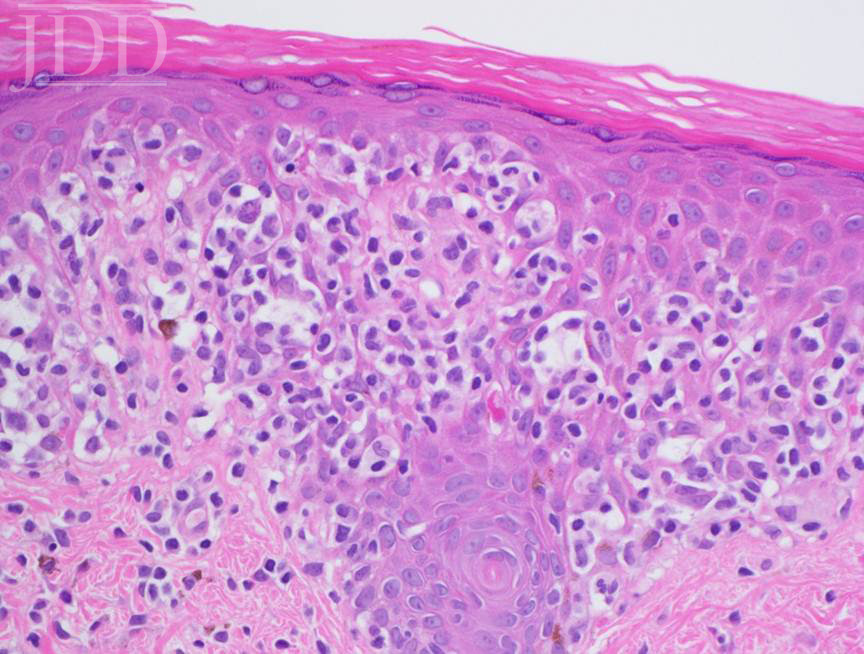
Lesional skin biopsy of a hypopigmented macule demonstrated an atypical superficial dermal band-like CD8 predominant T-cell infiltrate with prominent epidermotropism. The atypical lymphoid cells have irregular nuclear contours, hyperchromatic and enlarged nuclei. The atypical epidermotropic T-cells are immunoreactive for CD2, CD3, CD7 (partial), CD8, T-cell receptor beta F1 and non-immunoreactive for CD30 and T-cell receptor gamma delta. Diminished CD7 staining is noted within this atypical lymphoid infiltrate. Scattered reactive CD4-positive T-cells are present within the epidermis and superficial dermis. Molecular studies for T-cell receptor gene rearrangement are equivocal due to minimal amounts of DNA (limited template). The constellation of the morphologic, immunophenotypic and molecular genetic findings are compatible with mycosis fungoides. Peripheral blood flow cytometry, performed as part of her staging, is negative. Narrowband UVB and topical triamcinolone were recommended.

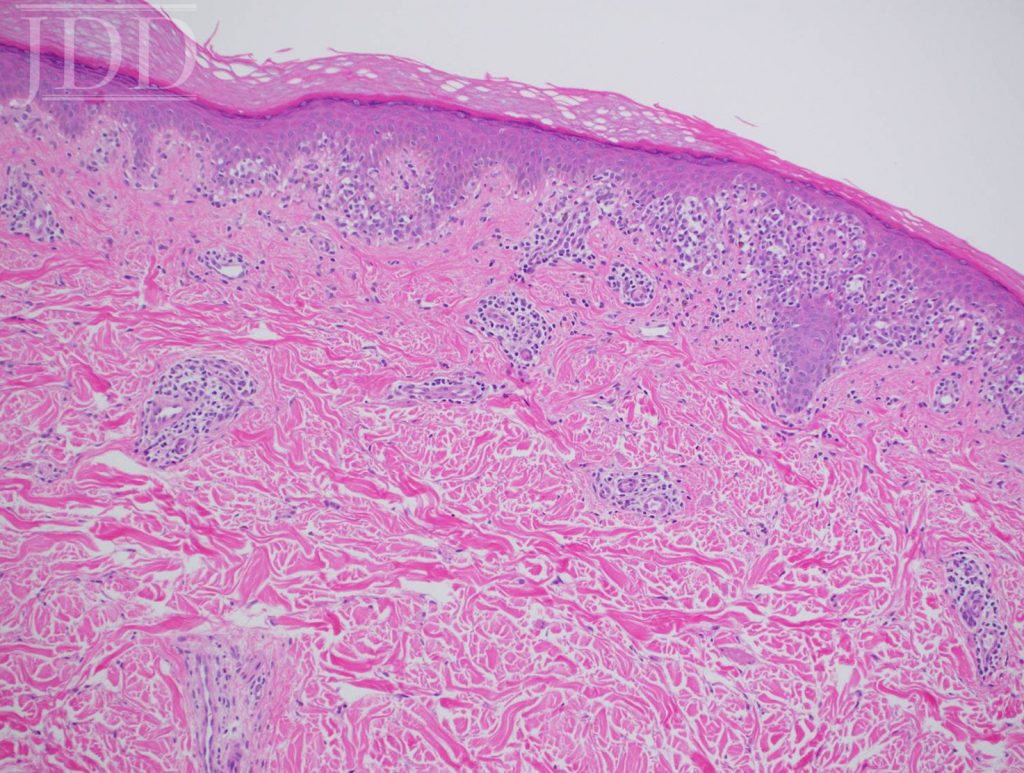
infiltrate with epidermotropism.
DISCUSSION
Hypopigmented Mycosis Fungoides (HMF) is a rare variant of cutaneous T-cell lymphoma (CTCL) that clinically presents as hypopigmented macules and patches typically on the extremities. As evident in this patient’s history, it is often mistaken for vitiligo, tinea versicolor, or pityriasis alba.³ HMF largely affects darker complected, young individuals in their early 20’s; however, the age range in 2 large studies is quite broad from 4 to 45 years old.2,4 The indolent behavior of HMF and its frequent misdiagnosis contributes to a latency period between appearance of lesions and diagnosis averaging 9.2 years. The definitive diagnosis often depends on histopathologic interpretation after clinical suspicion; therefore, a lesional biopsy is crucial.
Histopathologic features include epidermotropism of single or clustered, atypical, lymphocytes with hyperchromatic nuclei and irregular contours.5 CD8+ T cells are more prominent proportionally;6 however, this is not always the case. This finding is in contrast to the CD4+ T-cell predominance observed in classic MF. Clonal T-cell receptor gene rearrangement is frequently observed, while lack of clonality does not rule out HMF.7 Other immunohistochemical tests have been proposed, including decreased expression of CD117, similar to vitiligo,8 and negative staining for CD20, CD30, and CD79. The atypical cells can also stain positively for CD3, CD4, CD5, CD8, CD43, and Ki67.5
In contrast to vitiligo, her previous diagnosis and mimicker of her disease, HMF has prominent epidermotropism, hydropic degeneration of basal cells, partial pigment loss, preservation of some melanocytes, lymphocyte presence in papillary dermis, increased density of dermal infiltrate, and wiry fibrosis of the papillary dermal collagen.7 Pityriasis alba, tinea versicolor, leprosy, sarcoid, post-inflammatory hypopigmentation, and vitiligo-like inflammatory processes may simulate HMF clinically,5,7 and treatment failure of HMF to standard therapy should raise a suspicion of false-positive diagnosis of HMF.9,10
The course of HMF is similar to classic early patch stage MF and is usually indolent with an extended latent period before diagnosis. Initial evaluation may include a complete blood count, complete metabolic panel, T and B-cell lymphoid flow cytometry studies, and a chest X-ray. With appropriate treatment, most patients experience complete response; however, recurrence can occur.4 While no standard treatment is outlined, topical corticosteroids, narrowband UVB, and PUVA have all been successful first line treatment modalities.4 Topical mechlorethamine and carmustine have also been reported to be effective.1,5
This patient’s presentation illustrates well the difficulty in diagnosing hypopigmented mycosis fungoides. A latency period from the initial lesions to diagnosis is probably due to the disease rarity, indolence, mimicry, and underreporting.³ Even after obtaining a biopsy, the diagnosis is difficult to make, and response to treatment should be monitored. The favorable prognosis, depending on extent of the disease and co-morbidities, may be conveyed to the patient; however, the chance of recurrence should be outlined in the expectations.
REFERENCES
-
- Hassab-El-Naby HM, El-Khalawany MA. Hypopigmented mycosis fungoides in Egyptian patients. Journal of cutaneous pathology. Apr 2013;40(4):397-404.
- Castano E, Glick S, Wolgast L, et al. Hypopigmented mycosis fungoides in childhood and adolescence: a long-term retrospective study. Journal of cutaneous pathology. Nov 2013;40(11):924-934.
- Whitmore SE, Simmons-O’Brien E, Rotter FS. Hypopigmented mycosis fungoides. Archives of dermatology. Apr 1994;130(4):476-480.
- Akaraphanth R, Douglass MC, Lim HW. Hypopigmented mycosis fungoides: treatment and a 6(1/2)-year follow-up of 9 patients. Journal of the American Academy of Dermatology. Jan 2000;42(1 Pt 1):33-39.
- Zhang JA, Yu JB. Hypopigmented mycosis fungoides in a chinese woman. Indian journal of dermatology. Mar 2013;58(2):161.
- El-Shabrawi-Caelen L, Cerroni L, Medeiros LJ, McCalmont TH. Hypopigmented mycosis fungoides: frequent expression of a CD8+ T-cell phenotype. The American journal of surgical pathology. Apr 2002;26(4):450-457.
- El-Darouti MA, Marzouk SA, Azzam O, et al. Vitiligo vs. hypopigmented mycosis fungoides (histopathological and immunohistochemical study, univariate analysis). European journal of dermatology : EJD. Jan-Feb 2006;16(1):17-22.
- Singh ZN, Tretiakova MS, Shea CR, Petronic-Rosic VM. Decreased CD117 expression in hypopigmented mycosis fungoides correlates with hypomelanosis: lessons learned from vitiligo. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. Sep 2006;19(9):1255-1260.
- Petit T, Cribier B, Bagot M, Wechsler J. Inflammatory vitiligo-like macules that simulate hypopigmented mycosis fungoides. European journal of dermatology: EJD. Jul-Aug 2003;13(4):410-412.
- Werner B, Brown S, Ackerman AB. “Hypopigmented mycosis fungoides” is not always mycosis fungoides! The American Journal of dermatopathology. Feb 2005;27(1):56-67.
Source:
Tolkachjov, S. N., & Comfere, N. I. (2015). Hypopigmented mycosis fungoides: a clinical mimicker of vitiligo. J Drugs Dermatol, 14(2), 193-194.
Content and images used with permission from the Journal of Drugs in Dermatology.
Adapted from original article for length and style.

