JDD Authors Rachel Fayne BA, Sonali Nanda MS, Anna Nichols MD PhD, and John Shen MD report a case of biopsy-proven invasive SCC in an 86-year-old Caucasian male with history of multiple actinic keratoses and no previous skin cancers. The patient declined surgical treatment due to concerns about cosmetic outcomes. A combination of topical 5% imiquimod cream, 2% 5-FU solution, and 0.1% tretinoin cream was used five nights per week under occlusion for a treatment goal of 30 total applications. The patient was evaluated in clinic every 2 weeks during which the site was treated with cryotherapy. The patient reported burning pain associated with treatment and only completed 24 of the 30 applications.
INTRODUCTION
CASE
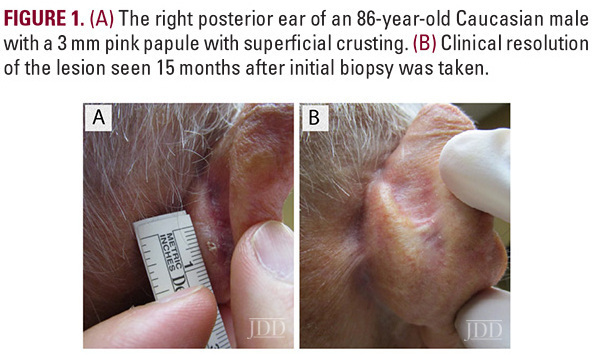
An 86-year-old Caucasian male with a previous history of actinic keratoses on
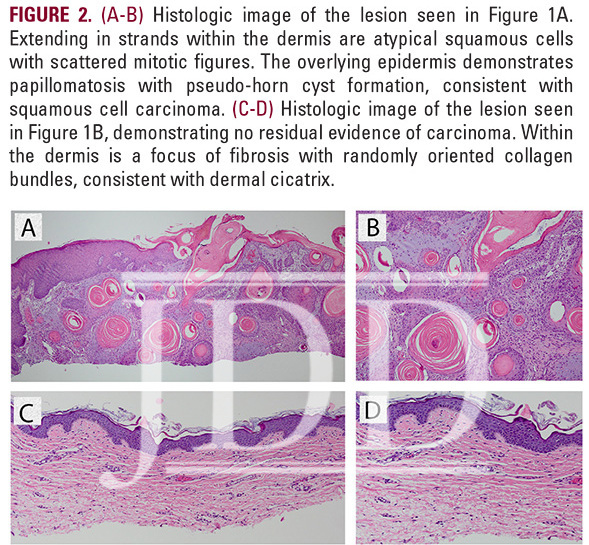
the scalp, and no prior history of skin cancer, presented with a tender, crusted pink papule, approximately 3 mm in diameter, on his right posterior ear (Figure 1A). Pathology confirmed invasive SCC described as atypical squamous cells with scattered mitotic figures extending in strands within the dermis. The epidermis demonstrated papillomatosis with pseudo-horn cyst formation (Figure 2A-B). The patient was extremely concerned with the cosmetic outcome of surgical treatment and sought alternative options, thus a non-surgical treatment plan was employed using a combination of off-label topical treatments used frequently as monotherapy for superficial BCC or SCC in situ in patients who defer surgery. The treatment goal was 30 total applications of 2% 5-FU solution, 5% imiquimod cream, and 0.1% tretinoin cream under occlusion 5 nights per week. Every two weeks the patient was evaluated in clinic and the lesion was treated with local cryotherapy at a distance of 6 mm from the lesion for 1 second and with 1 freeze-thaw cycle. After 24 treatments, the patient developed burning pain on his posterior ear and discontinued treatment.
DISCUSSION
Skin cancer is the most common cancer in the United States, with approximately 9,500 cases diagnosed every day,8,9 and its incidence is on the rise.10 SCC is associated with very low mortality if detected and treated early, with standard of care being surgical excision or Mohs micrographic surgery.1 However, not all patients are ideal surgical candidates. Older patients are a particularly important population to consider, given that increased age is a known risk factor for the development of SCC.1 Although it has been shown that this population can safely undergo Mohs micrographic surgery,11 it is well-known that elderly patients suffer from slower wound healing.12 One recent study shows that approximately one third of patients over 80 and one half of patients over 90 had no residual carcinoma after initial biopsy. Taking this into account along with relative patient longevity, age may be a significant consideration before excision of facial non-melanoma skin cancers, including SCC.13 Further, many patients accumulate numerous skin,1 cancers in their lifetime, often on the face and other cosmetically sensitive areas and may not want to undergo invasive procedures for every lesion. Surgical management may also leave patients with unwanted scars, which has been demonstrated to significantly affect psychosocial functioning.14 This was of particular concern for our patient. Thus, there is a need for non-invasive treatment options. Current non-surgical options for the treatment of SCC include PDT, cryotherapy, and RT.3 Topical medications like imiquimod and 5-FU have been studied off-label for SCC in situ, and, in several small reports, invasive SCC.
Imiquimod acts at the level of the toll-like receptors (TLRs) on antigen-presenting cells, initiating a cascade of events that leads to increased production of IFN-γ, a potent inhibitor of angiogenesis, and Fas receptor-ligand pro-apoptotic signaling, which culminates in caspase activation and cell death.15 Imiquimod has been studied for the treatment of SCC in situ, and, in several small case series for the treatment of invasive SCC. In one case series, 4 patients with SCC in situ and 6 with invasive or recurrent invasive SCC underwent treatment with 5% imiquimod cream 5 times per week for 16 weeks. Of the 6 patients with invasive SCC, 4 demonstrated complete regression and 2 demonstrated partial regression by the end of treatment and without recurrence for a mean follow-up period of 31 months.16 In another, two patients with SCC in situ and one with invasive SCC were treated with 5% imiquimod cream nightly for six weeks, and follow-up biopsy demonstrated no residual carcinoma or carcinoma in situ.17
5-FU functions as an antimetabolite, binding to thymidylate synthetase and inhibiting conversion of deoxyuridine to thymidine. This targets highly mitotic, neoplastic cells, reducing DNA synthesis and leading to cell death.15 Topical 5-FU has not yet been reported for the treatment of invasive SCC or SCC in situ. Intralesional and topical 5-FU is often used to treat keratoacanthomas, 18,19 a variant of SCC, and is approved for the treatment of superficial BCC.2,3
Both imiquimod and 5-FU commonly induce local side effects, including erythema and symptoms of burning, pain, and itching. Rarely, imiquimod can induce like flu-like symptoms.20,21 Despite these known side effects, many patients still report a preference of topical therapy over surgery.22In addition to patient preference and avoiding known potential surgical complications, topical therapy is also less costly to the patient.23
Because topical imiquimod and 5-FU are not approved for SCC in situ or invasive SCC, data regarding long-term tumor-free survival is limited. Imiquimod and 5-FU are approved treatments for superficial basal cell carcinoma (BCC), and studies have shown that individually, these topical therapies may yield significant long-term tumor-free survival rates for superficial BCC, but they are still less effective than surgery when used as monotherapy. 4,5Because these two drugs act through distinct mechanisms of action, combination therapy is a reasonable means to optimize outcomes for patients who wish to avoid surgery.21
The third topical agent used, tretinoin, is known to interfere with carcinogenesis and apoptosis. It is a retinoid, or a vitamin-A derivative, that acts on nuclear retinoic acid receptors in order to control cellular proliferation and differentiation.15 Topical tretinoin has been studied for its potential role as a chemopreventive agent for BCC and SCC, but thus far has been proven ineffective [24]. One study in renal transplant patients, with increased risk for SCC, showed that a combination of topical tretinoin and low dose systemic retinoids, in an attempt to lessen toxicity associated with high dose systemic retinoids, reduced the number of existing SCCs and reduced risk of new SCCs.25 While efficacy of topical tretinoin as monotherapy for SCC has not been studied, a combination of topical tretinoin, imiquimod and 5-FU, each with their distinct mechanisms of action, may provide improved long-term tumor free survival compared to single-drug regimens for patients with invasive SCC.
We present a patient with invasive SCC successfully treated with combination topical therapy consisting of 5% imiquimod cream, 2% 5-FU solution, and 0.1% tretinoin cream. Previous reports of the use of single-drug topical therapy with imiquimod or 5-FU in the treatment of SCC in situ shown promising results, however the success rates were still lower than surgical management. We demonstrate that the combination of these three medications provided an excellent outcome in our case, although the follow-up was only 25 months. Prospective randomized clinical trials are needed to support these findings, which may offer patients a non-surgical alternative to the current standard of care.
REFERENCES
1. Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379(4):363-374.
2. Chitwood K, J. Etzkorn, and G. Cohen, Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons. Dermatol Surg. 2013;39(9):1306-16.
3. Collins, A., J. Savas, and L. Doerfler. Nonsurgical treatments for nonmelanoma skin cancer. Dermatol Clin. 2019. 37(4):435-441.
4. Williams, H.C., et al., Surgery versus 5% imiquimod for nodular and superficial basal cell carcinoma: 5-year results of the SINS randomized controlled trial. J Invest Dermatol. 2017. 137(3):614-619.
5. Jansen, M.H.E., et al., Five-Year results of a randomized controlled trial comparing effectiveness of photodynamic therapy, topical imiquimod, and topical 5-fluorouracil in patients with superficial basal cell carcinoma. J Invest Dermatol. 2018. 138(3): p. 527-533.
6. Quirk, C., et al., Sustained clearance of superficial basal cell carcinomas treated with imiquimod cream 5%: results of a prospective 5-year study. Cutis. 2010. 85(6): p. 318-24.
7. Bath-Hextall, F., et al., Surgical excision versus imiquimod 5% cream for nodular and superficial basal-cell carcinoma (SINS): a multicentre, non-inferiority, randomised controlled trial. Lancet Oncol. 2014. 15(1):96-105.
8. Guy, G.P., Jr., et al., Vital signs: melanoma incidence and mortality trends and projections – United States, 1982-2030. MMWR Morb Mortal Wkly Rep, 2015. 64(21): p. 591-6.
9. Rogers, H.W., et al., Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015. 151(10):1081-6.
10. Muzic, J.G., et al., Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: a population-based study in olmsted county, Minnesota, 2000 to 2010. Mayo Clin Proc. 2017. 92(6):890-898.
11. Regula, C.G., et al., Functionality of patients 75 years and older undergoing mohs micrographic surgery: a multicenter study. Dermatol Surg. 2017. 43(7):904-910.
12. Rogers EM, et al. Comorbidity scores associated with limited life expectancy in the very elderly with nonmelanoma skin cancer. J Am Acad Dermatol. 2018. 78(6):1119-1124.
13. Chauhan R, et al. Age at Diagnosis as a relative contraindication for intervention in facial nonmelanoma skin cancer. JAMA Surg. 2018. 153(4):390-392.
14. Sobanko, JF, et al. Importance of physical appearance in patients with skin cancer. Dermatol Surg. 2015. 41(2):183-8.
15. Micali G, et al. Topical pharmacotherapy for skin cancer: part I. Pharmacology. J Am Acad Dermatol. 2014. 70(6):965 e1-12; quiz 977-8.
16. Peris K, et al., Imiquimod treatment of superficial and nodular basal cell carcinoma: 12-week open-label trial. Dermatol Surg. 2005. 31(3):318-23.
17. Nouri K, C. O’Connell, and M.P. Rivas, Imiquimod for the treatment of Bowen’s disease and invasive squamous cell carcinoma. J Drugs Dermatol. 2003. 2(6): p. 669-73.
18. Goette DK. Treatment of keratoacanthoma with topical fluorouracil. Arch Dermatol. 1983. 119(11):951-3.
19. Kiss N, et al. Intralesional therapy for the treatment of keratoacanthoma. Dermatol Ther. 2019. 32(3):e12872.
20. Arits AH, et al. Photodynamic therapy versus topical imiquimod versus topical fluorouracil for treatment of superficial basal-cell carcinoma: a single blind, non-inferiority, randomised controlled trial. Lancet Oncol. 2013. 14(7):647-54.
21. Shaw FM and M.A. Weinstock, Comparing topical treatments for basal cell carcinoma. J Invest Dermatol. 2018. 138(3):484-486.
22. Tinelli M, et al. What determines patient preferences for treating low risk basal cell carcinoma when comparing surgery vs imiquimod? A discrete choice experiment survey from the SINS trial. BMC Dermatol. 2012. 12:19.
23. Yoon J, et al. Impact of topical fluorouracil cream on costs of treating keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis. J Am Acad Dermatol. 2018. 79(3): p. 501-507 e2.
24. Weinstock MA, et al. Tretinoin and the prevention of keratinocyte carcinoma (Basal and squamous cell carcinoma of the skin): a veterans affairs randomized chemoprevention trial. J Invest Dermatol. 2012. 132(6):1583-90.
25. Rook AH, et al., Beneficial effect of low-dose systemic retinoid in combination with topical tretinoin for the treatment and prophylaxis of premalignant and malignant skin lesions in renal transplant recipients. Transplantation. 1995. 59(5):714-9.
Source:
Rachel Fayne BA, Sonali Nanda MS, Anna Nichols MD PhD, John Shen MD (2020). Combination Topical Chemotherapy for the Treatment of an Invasive Cutaneous Squamous Cell Carcinoma. Journal of Drugs in Dermatology, 19 (2). https://jddonline.com/articles/dermatology/S1545961620P0202X
Content and images used with permission from the Journal of Drugs in Dermatology.
Adapted from original article for length and style.
Did you enjoy this case report? Find more here.
Next Steps in Derm is brought to you by SanovaWorks.