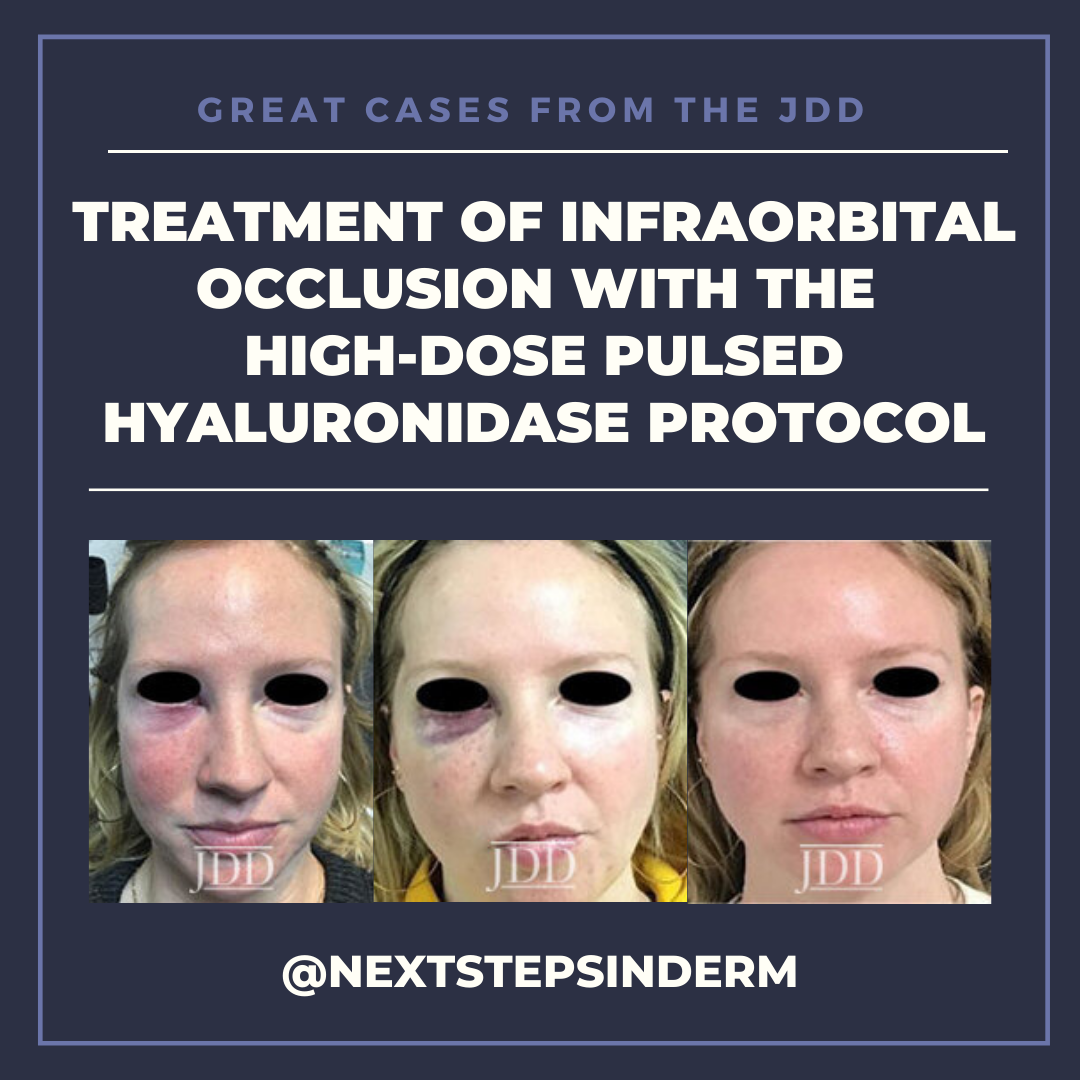
CASE
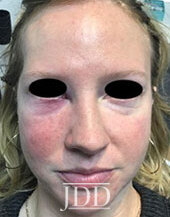
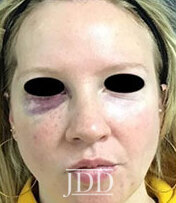
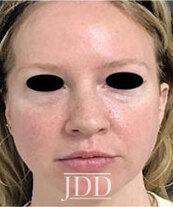
DISCUSSION
The patient’s skin findings during filler injection were consistent with infra-orbital arterial occlusion. Our report of high dose HYAL administered hourly provided remarkable improvement.
HA fillers, lauded for their safety and relative longevity, also have the advantage of reversibility with HYAL, a naturally occurring enzyme that degrades the product. Thus, HYAL is an essential component of the aesthetic dermatologist’s arsenal, aiding in the management and correction of intravascular HA-filler related cutaneous complications.1 HYAL is typically injected directly into the affected site of the previously injected HA, followed usually by massage to facilitate further hydrolysis.1
The high dose pulse HYAL injection protocol, described by DeLorenzi, is based on the principle that arteries obstructed with HA filler need to be flooded with sufficiently high concentration of HYAL for a long enough time to dissolve the filler and relieve the obstruction of HA.2 This flooding, the author suggests, is necessary because partial or inadequate hydrolysis of the occlusion may cause smaller breakdown products to travel downstream and cause further obstruction.2Prior to this regimen, earlier treatment protocols included lower doses of HYAL, aspirin, topical nitroglycerin, warm compresses, and hyperbaric oxygen. The recommended dosage of HYAL increases in a stepwise fashion as the number of anatomical areas involved increases which is estimated by clinical signs of ischemic damage such as pain, poor capillary refill, and livedo reticularis.2 Repeated doses on an hourly basis are recommended because HYAL is being continuously removed from the bloodstream via dilution, diffusion, and degradation, and thus, needs to be replenished regularly. Success is reported to be common when treatment is completed within 72 hours of onset of ischemia.2
Few published reports have described similar methods to treat suspected occlusion with success. Loh et al reported the treatment of a right infraorbital arterial occlusion with three 1000-unit pulses of high-dose pulsed HYAL, administered hourly.3 Chauhan et al also treated the right infraorbital artery vascular territory with 3 dosed pulse of 500 units after the patient presented 48 hours post-injection with complaints of pulsating pain in the right infraorbital and nasolabial area.4 This delayed presentation was also treated with an additional 500 units the following day.
The use of cannulas for filler injection is thought to be safer than needles due to its blunt edge and the increased force required for intra-arterial penetration, thereby theoretically reducing the risk of vascular occlusion. However, one cadaveric study demonstrated that the 27 G cannula was no safer than a 27 G needle.5 In our case, a 27 G cannula was used by the injector prior to the occlusion. Thus, based on data from other published reports, use of cannulas 25 G or larger in size may be prudent. In addition, aspiration does not guarantee a “safe” injection and injector knowledge of facial anatomy is critical not only while injecting but demarcating the area of vascular involvement in the case of occlusion. Familiarity with clinical signs of vascular obstruction is key as well as being equipped in the office to respond quickly. Anesthetics included in the filler can mask pain until metabolized, underscoring the need to be vigilant for other often more subtle signs.2
Vascular compromise due to filler injections, while uncommon, will likely increase as the popularity of filler treatments increases. Despite this, there is a paucity of literature on the successful treatment of such vascular compromise. Therefore, this case demonstrates the efficacy of high dose pulsed HYAL for the treatment of vascular compromise during injection with HA filler.
REFERENCES
2. DeLorenzi C. New high dose pulsed hyaluronidase protocol for hyaluronic acid filler vascular adverse events. Aesthet Surg J. 2017;37:814-25.
3. Loh, KTD, Phoon YS, Phua V, Kapoor KM. successfully managing impending skin necrosis following hyaluronic acid filler injection, using high-dose pulsed hyaluronidase. Plast Reconstr Surg- Glob Open. 2018;6:e1639.
4. Singh S, Chauhan A. Management of delayed skin necrosis following hyaluronic acid filler injection using pulsed hyaluronidase. J Cutan Aesthet Surg. 2019; 12:183.
5. Pavicic T, et al. Arterial wall penetration forces in needles versus cannulas. Plast Reconstr Surg. 2019;143:504e-12e.
SOURCE
Content and images used with permission from the Journal of Drugs in Dermatology.
Adapted from original article for length and style.