ABSTRACT
The risk of post-inflammatory hyperpigmentation (PIH) in patients undergoing dermatologic procedures is well known. It is especially common after laser procedures and chemical peels but can be seen with any procedure. PIH is also a sequela of acne, burns, and other trauma. High-risk patients are thought to have excessive production and abnormal distribution of melanin within the skin that triggers PIH, but the exact pathophysiology is unknown.1 We define high-risk patients as Fitzpatrick skin types 3–5, those with existing PIH, or a history of PIH.1,2
Tranexamic acid (TXA) is an antifibrinolytic medication prescribed to treat bleeding and is also used off-label to treat melasma. TXA is contraindicated in patients with hypercoagulable conditions, renal impairment, vision impairment disorders, pregnancy, breast-feeding, or on hormone therapies.3,4,5
From 2015–2020, we have used TXA off-label to successfully treat and/or prevent PIH in approximately 82 high-risk patients after injuries or prior to procedures that disrupt the epidermis. We also have used TXA to prevent PIH after acute injuries such as irritant dermatitis, thermal burns, and abrasions. We now consider TXA treatment for all at risk patients prophylactically before undergoing microneedling, cryotherapy, cryolipolysis, chemical peels, and laser treatments.
CASE 1
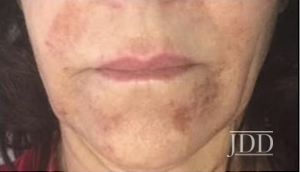
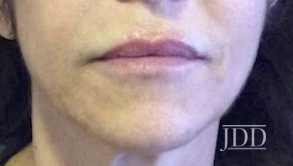
CASE 2
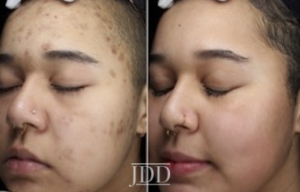
DISCUSSION
Skin dyschromias such as melasma, lentigos, and PIH are dermatologic conditions that can have significant negative impacts on the quality of life of affected patients.1,2 Ironically, many of the procedures that treat pigment disorders can also cause irritation with the subsequent worsening or development of PIH. Prophylactic use of sunscreens, topical corticosteroids, and topical depigmenting agents have been used in an attempt to prevent and treat PIH with varying success.2 PIH with or without treatment can take many months or even years to fully resolve. We believe this is because established topical treatments cannot be used after injury until the skin has re-epithelialized and the pathogenesis of hyperpigmentation has already been set in motion. TXA can be administered orally immediately after injury. Once the skin is healed, all patients are also counseled to use sun protection and established topical treatments2 may be used in combination with TXA. The exact mechanism of TXA in reducing melanogenesis is unknown. It blocks melanocyte and keratinocyte interactions by inhibiting plasmin and mimicking lysine to prevent plasminogen from binding to keratinocytes. This decreases the inflammatory arachidonic acid and prostaglandins that stimulate melanocytes.5,6,7 TXA is structurally similar to tyrosinase and may work by competitively antagonizing the enzyme.7 TXA inhibits ultraviolet induced plasmin activity and reduces ultraviolet melanogenesis.3,7,8 TXA has been shown to inhibit melanogenesis through activating autophagy in cultured melanoma cells.8 Kim et al showed TXA treated melanocytes exhibited decreased melanin content and tyrosinase activity in lasered cells, adding to the rationale for using TXA to prevent or treat PIH after procedures.9
Three studies have looked at TXA for the prevention of PIH. TXA 650 mg daily was given to 32 Japanese women before Q-switched ruby laser (QSRL) for the treatment of senile lentigos (SL). This prospective randomized study found no significant difference between the treatment and placebo group with regard to PIH. This protocol may have been suboptimal due to small sample size, TXA dose, or duration of treatment.3 Another prospective randomized study looked at the effect of 1500 mg daily of oral TXA on 40 patients undergoing SL treatment with Q-switched 532‐nm Nd:YAG laser. This dose was not effective in preventing PIH compared with placebo. However, oral TXA did speed resolution of PIH when continued up to six weeks posttreatment posttreatment.4 The third study found a single dose of intradermal TXA injection (50 mg/mL) was mildly successful in reducing PIH after SL removal with a Q-switched 532-nm Nd:YAG laser when compared with placebo (0.9% normal saline) injection.5 We use oral TXA at a dose of 650 mg daily until the injury is fully healed and no PIH is present. This time period ranges from 2 weeks to several years in the case of ongoing treatment. We counsel patients to stop treatment for 48 hours prior to periods of immobility such as air or car flights over 4 hours and to avoid dehydration.
A recent extensive review and meta-analysis of nonsurgical patients treated with TXA without known hypercoagulability or risk factors for hypercoagulability such as drug interactions, active bleeding, cancer, past blood clots, or hormone therapy showed no increased risk of venous or arterial blood clots.10
A randomized, controlled study of acute TXA treatment to prevent and treat PIH would be extremely difficult to undertake given the individual variation of its expression, even within the same individual with the same type of injury. Until alternative studies can be completed, adding oral TXA to one’s armamentarium in the setting of acute skin injury to prevent PIH in at-risk patients is safe and appears effective.
REFERENCES
-
- Passeron T, Nouveau S, Duval C, et al. Development and validation of reproducible model for studying post-inflammatory hyperpigmentation. Pigment Cell Melanoma Res. 2018;31(5):649-652.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3(7):20-31.
- Kato H, Araki J, Eto H, et al. A prospective randomized controlled study of oral tranexamic acid for preventing postinflammatory hyperpigmentation after Q-switched ruby laser. Dermatol Surg. 2011;37(5):605-610.
- Rutnin S, Pruettivorawongse D, Thadanipon K, Vachiramon V. A prospective randomized controlled study of oral tranexamic acid for the prevention of postinflammatory hyperpigmentation after Q-switched 532-nm Nd:YAG laser for solar lentigines. Lasers Surg Med. 2019;51(10):850-858.
- Sirithanabadeekul P, Srieakpanit R. Intradermal tranexamic acid injections prevent post-inflammatory hyperpigmentation after solar lentigo removal with a Q-switched 532-nm Nd:YAG laser. J Cosmet Laser Ther.2018;20(7- 8):398-404.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32(5):626-631.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg.2018;44(6):814-825.
- Cho YH, Park JE, Lim DS, Lee JS. Tranexamic acid inhibits melanogenesis by activating the autophagy system in cultured melanoma cells. J Dermatol Sci. 2017;88(1):96-102.
- Kim MS, Bang SH, Kim JH, et al. Tranexamic acid diminishes laser-induced melanogenesis. Ann Dermatol.2015;27(3):250-256.
- Chornenki NLJ, Um KJ, Mendoza PA, et al. Risk of venous and arterial thrombosis in non-surgical patients receiving systemic tranexamic acid: a systematic review and meta-analysis. Thromb Research. 2019;179:81-86.
Source
Lindgren, A. L., Austin, A. H., & Welsh, K. M. (2021). The Use of Tranexamic Acid to Prevent and Treat Post-Inflammatory Hyperpigmentation. Journal of Drugs in Dermatology: JDD, 20(3), 344-345.
Content and images used with permission from the Journal of Drugs in Dermatology.
Adapted from original article for length and style.
Did you enjoy this case report? Find more here.

