Rituximab-Induced Alopecia Universalis in a Patient With Bullous Pemphigoid
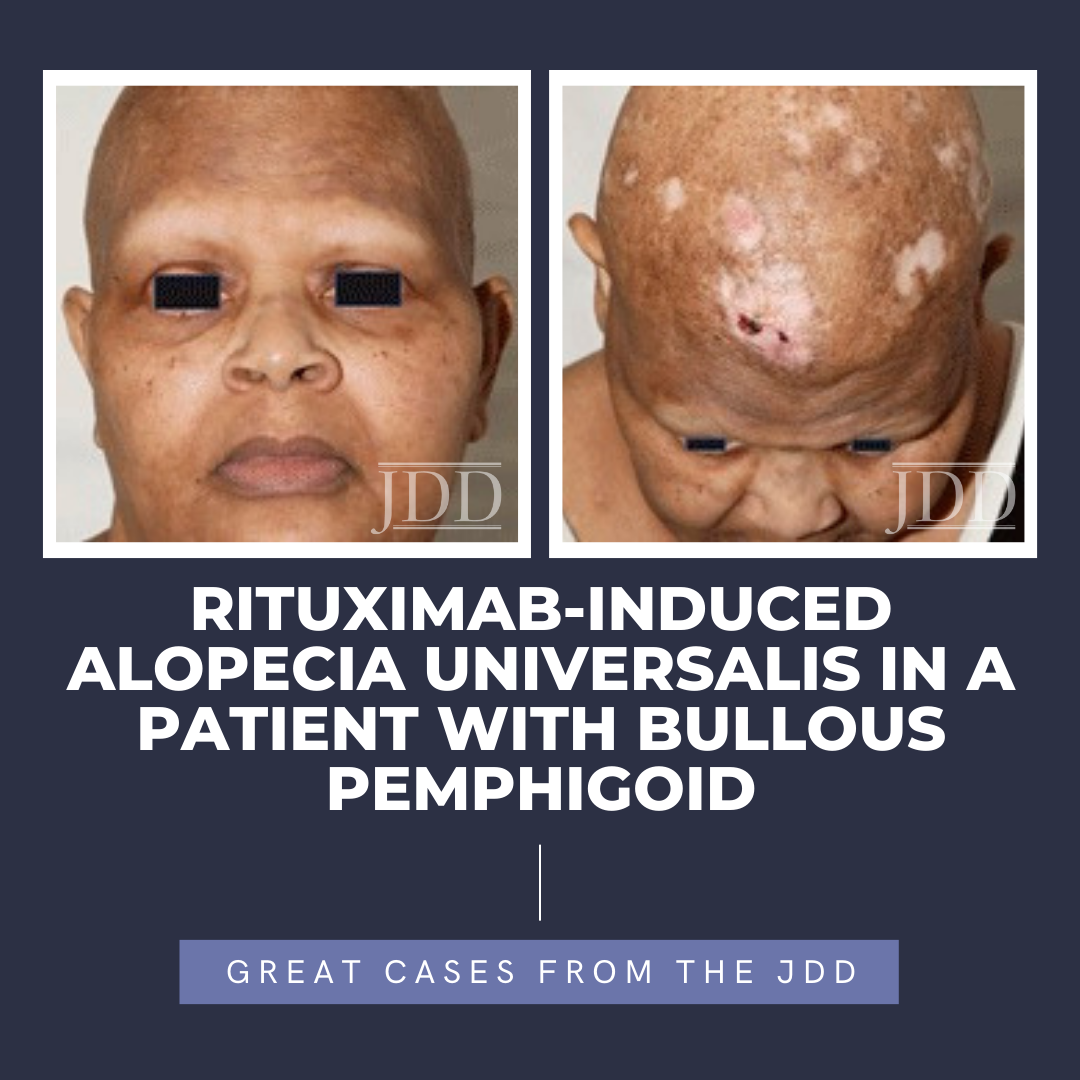 Alopecia areata is a CD8+ T-lymphocyte driven autoimmune disorder leading to reversible hair loss. While most commonly presenting as isolated well-demarcated non-cicatricial alopecic patches on the scalp, subtypes of alopecia areata include alopecia totalis with loss of all scalp hair and alopecia universalis with complete loss of all body hair. Although primarily an idiopathic condition, several …
Alopecia areata is a CD8+ T-lymphocyte driven autoimmune disorder leading to reversible hair loss. While most commonly presenting as isolated well-demarcated non-cicatricial alopecic patches on the scalp, subtypes of alopecia areata include alopecia totalis with loss of all scalp hair and alopecia universalis with complete loss of all body hair. Although primarily an idiopathic condition, several …
 Alopecia areata is a CD8+ T-lymphocyte driven autoimmune disorder leading to reversible hair loss. While most commonly presenting as isolated well-demarcated non-cicatricial alopecic patches on the scalp, subtypes of alopecia areata include alopecia totalis with loss of all scalp hair and alopecia universalis with complete loss of all body hair. Although primarily an idiopathic condition, several …
Alopecia areata is a CD8+ T-lymphocyte driven autoimmune disorder leading to reversible hair loss. While most commonly presenting as isolated well-demarcated non-cicatricial alopecic patches on the scalp, subtypes of alopecia areata include alopecia totalis with loss of all scalp hair and alopecia universalis with complete loss of all body hair. Although primarily an idiopathic condition, several … Continue reading "Rituximab-Induced Alopecia Universalis in a Patient With Bullous Pemphigoid"


 Next Steps in Derm, in partnership with Skin of Color Update, interviewed Dr. Shilpi Khetarpal, dermatologist at the Cleveland Clinic. Watch as Dr. Khetarpal outlines the four categories of adjunctive therapies for hair loss and why clinicians should pair them with traditional medical treatments. Learn Dr. Khetarpal’s typical treatment regimen when using PRP in hair loss patients. Find out what …
Next Steps in Derm, in partnership with Skin of Color Update, interviewed Dr. Shilpi Khetarpal, dermatologist at the Cleveland Clinic. Watch as Dr. Khetarpal outlines the four categories of adjunctive therapies for hair loss and why clinicians should pair them with traditional medical treatments. Learn Dr. Khetarpal’s typical treatment regimen when using PRP in hair loss patients. Find out what … 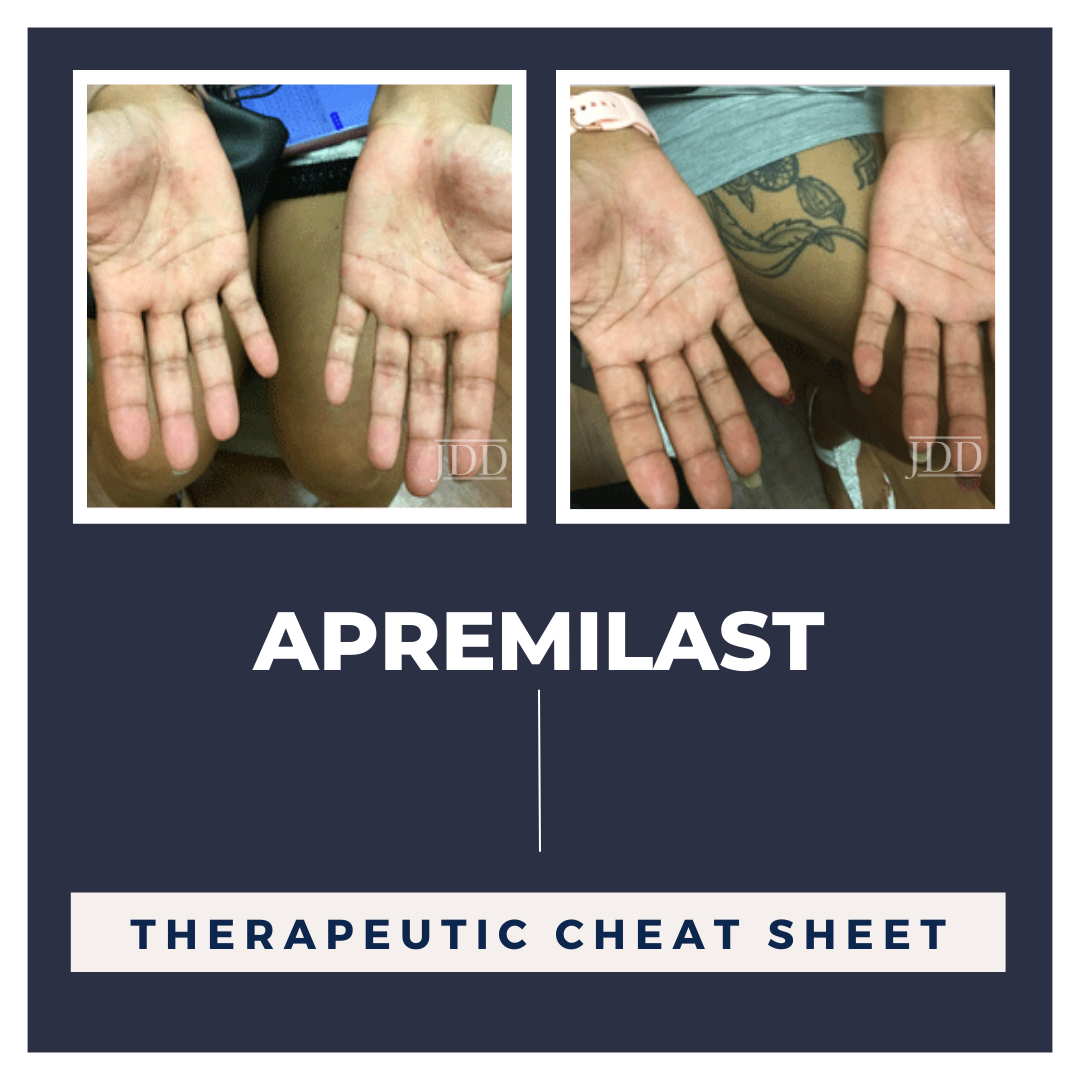 Apremilast (OTEZLA®) is a twice daily oral medication that is FDA approved for adults with plaque psoriasis, psoriatic arthritis and oral ulcers associated with Behçet’s Disease.1 This drug is being extended as an off-label treatment to target inflammation in a number of different conditions. This Therapeutic Cheat Sheet will focus on apremilast and its applications for different dermatologica …
Apremilast (OTEZLA®) is a twice daily oral medication that is FDA approved for adults with plaque psoriasis, psoriatic arthritis and oral ulcers associated with Behçet’s Disease.1 This drug is being extended as an off-label treatment to target inflammation in a number of different conditions. This Therapeutic Cheat Sheet will focus on apremilast and its applications for different dermatologica … 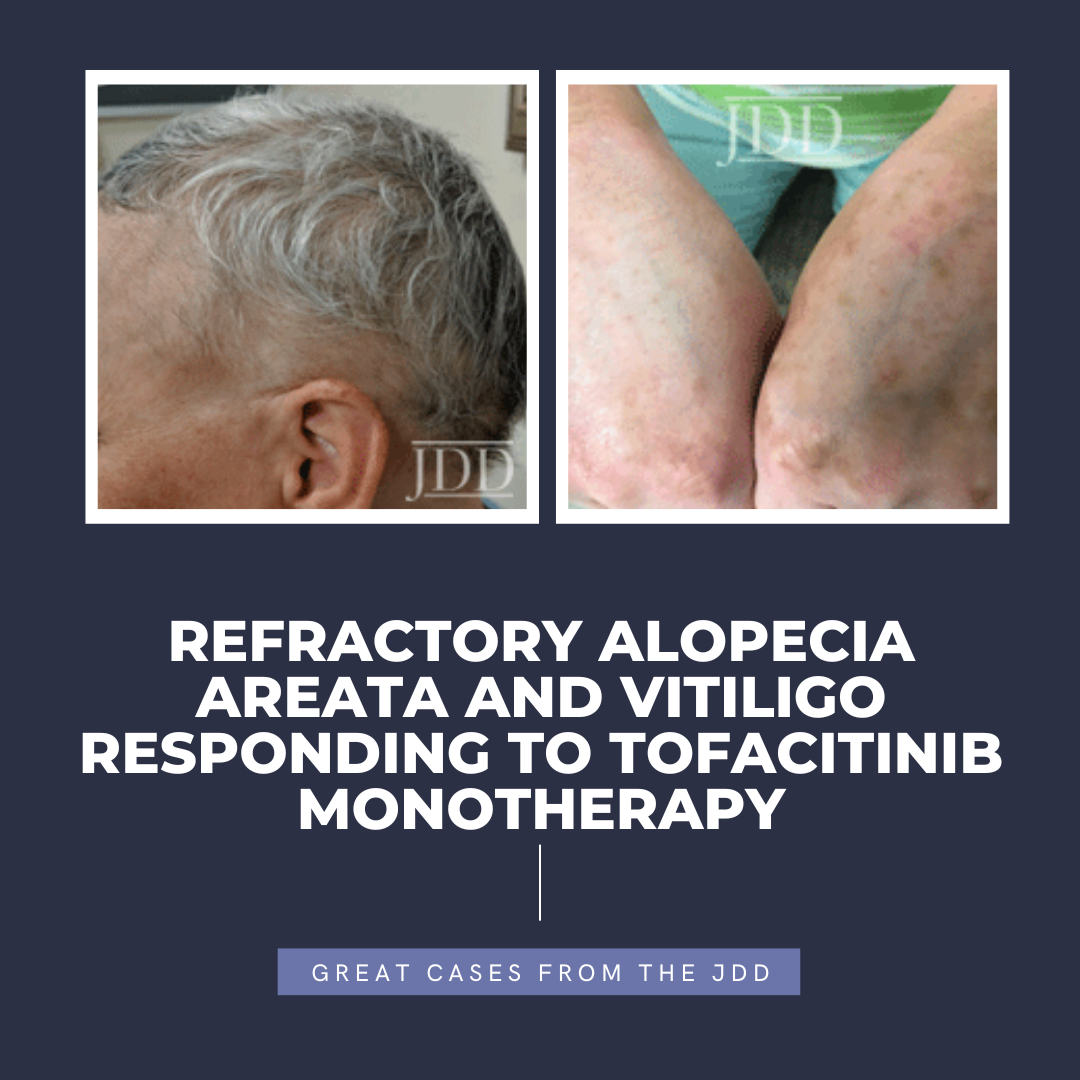 INTRODUCTION
Tofacitinib is a Janus kinase (JAK) 1-3 inhibitor first U.S. Food and Drug Administration (FDA) approved in 2012 for rheumatoid arthritis, with subsequent approval for psoriatic arthritis, ulcerative colitis, polyarticular course juvenile idiopathic arthritis, and ankylosing spondylitis in 2017, 2018, 2020, and 2021, respectively.1,2 In the last several years, oral tofacitinib …
INTRODUCTION
Tofacitinib is a Janus kinase (JAK) 1-3 inhibitor first U.S. Food and Drug Administration (FDA) approved in 2012 for rheumatoid arthritis, with subsequent approval for psoriatic arthritis, ulcerative colitis, polyarticular course juvenile idiopathic arthritis, and ankylosing spondylitis in 2017, 2018, 2020, and 2021, respectively.1,2 In the last several years, oral tofacitinib …  Upadacitinib (RINVOQ®) is a once daily oral medication that is FDA approved for a variety of conditions including psoriatic arthritis and atopic dermatitis. Jak inhibitors, like upadacitinib, are a class of drugs showing to be effective in treating inflammatory conditions. Upadacitnib is not only being used to treat rheumatoid arthritis and psoriatic arthritis, but was recently FDA approved i …
Upadacitinib (RINVOQ®) is a once daily oral medication that is FDA approved for a variety of conditions including psoriatic arthritis and atopic dermatitis. Jak inhibitors, like upadacitinib, are a class of drugs showing to be effective in treating inflammatory conditions. Upadacitnib is not only being used to treat rheumatoid arthritis and psoriatic arthritis, but was recently FDA approved i …