Friday Pop Quiz 9/6/2024
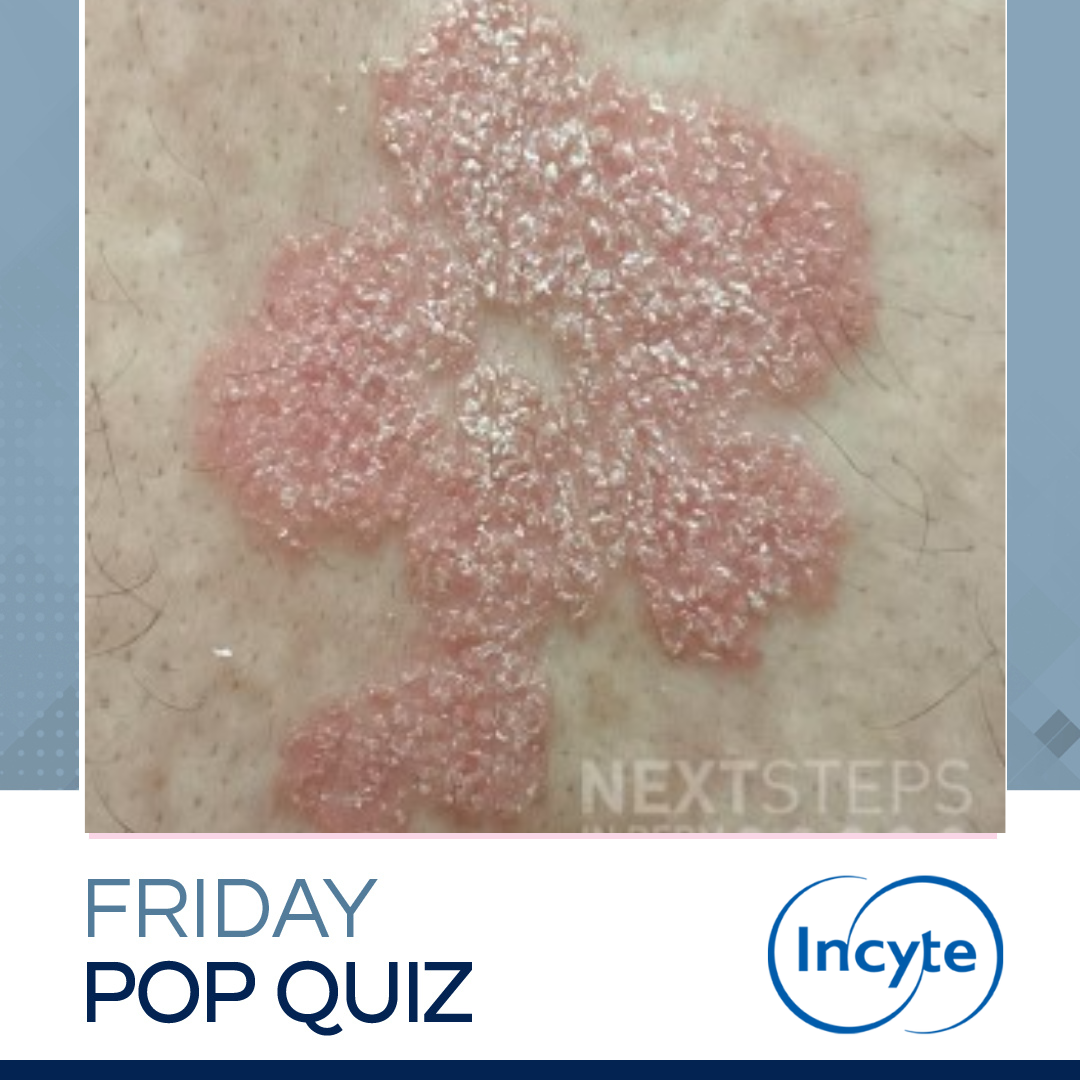 A 35-year-old woman presents to clinic with the skin lesions (shown) and joint pain. She was previously well controlled on guselkumab, but has flared since her dog was diagnosed with cancer. She has previously failed various treatments including topical steroids, methotrexate, narrow-band UVB, adalimumab, ixekizumab, and etanercept. She developed transaminitis and hepatic steatosis in response t …
A 35-year-old woman presents to clinic with the skin lesions (shown) and joint pain. She was previously well controlled on guselkumab, but has flared since her dog was diagnosed with cancer. She has previously failed various treatments including topical steroids, methotrexate, narrow-band UVB, adalimumab, ixekizumab, and etanercept. She developed transaminitis and hepatic steatosis in response t …
 A 35-year-old woman presents to clinic with the skin lesions (shown) and joint pain. She was previously well controlled on guselkumab, but has flared since her dog was diagnosed with cancer. She has previously failed various treatments including topical steroids, methotrexate, narrow-band UVB, adalimumab, ixekizumab, and etanercept. She developed transaminitis and hepatic steatosis in response t …
A 35-year-old woman presents to clinic with the skin lesions (shown) and joint pain. She was previously well controlled on guselkumab, but has flared since her dog was diagnosed with cancer. She has previously failed various treatments including topical steroids, methotrexate, narrow-band UVB, adalimumab, ixekizumab, and etanercept. She developed transaminitis and hepatic steatosis in response t … 

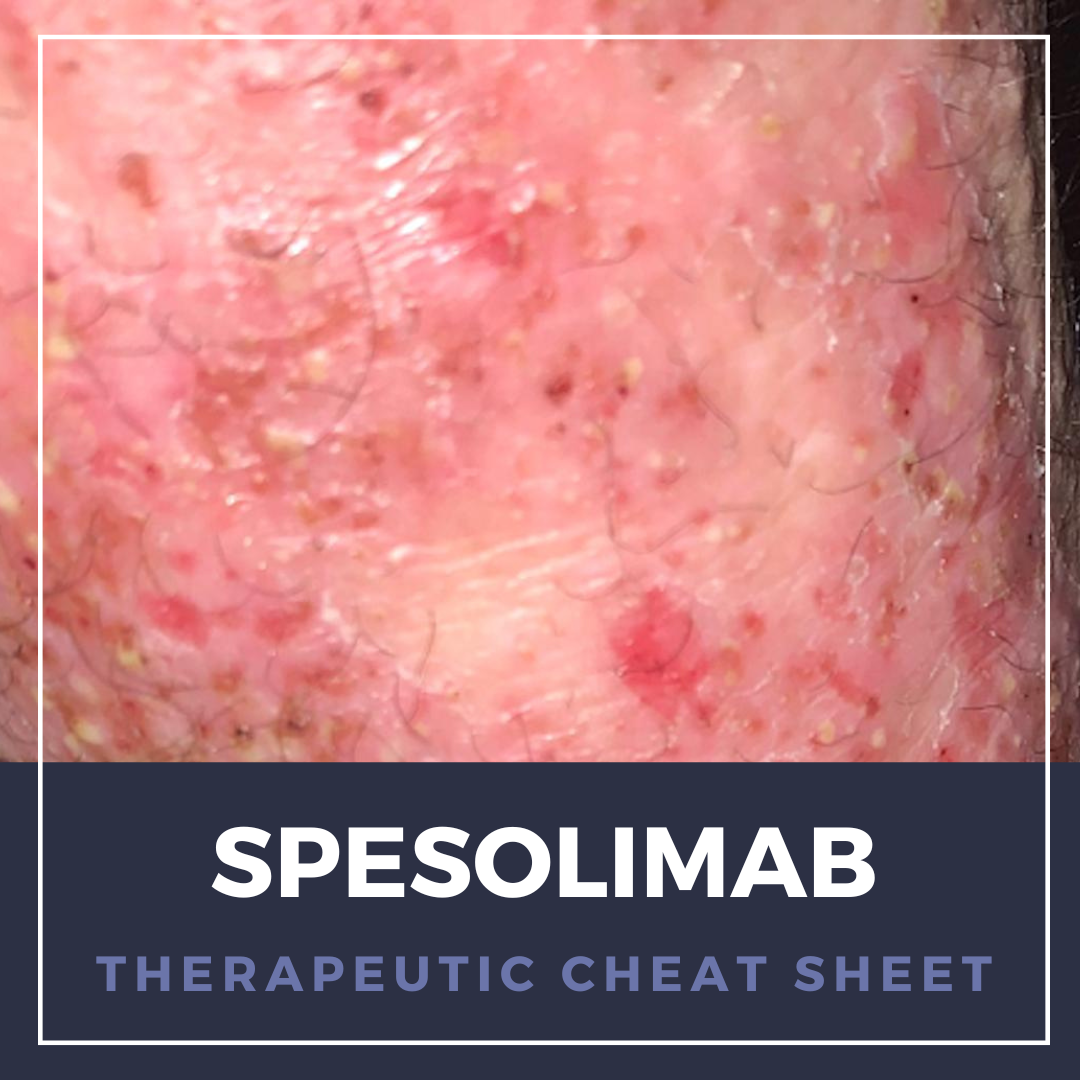 Generalized pustular psoriasis (GPP) is a rare, autoinflammatory condition, with acute, severe flares of diffuse sterile pustules and systemic symptoms that can be life-threatening. Historically, cyclosporine, methotrexate, retinoids, and biologics modulating the interleukin-17 (IL-17), IL-23, and tumor necrosis factor α pathways have been used off-label for management.1 Aberrant IL-36 signaling …
Generalized pustular psoriasis (GPP) is a rare, autoinflammatory condition, with acute, severe flares of diffuse sterile pustules and systemic symptoms that can be life-threatening. Historically, cyclosporine, methotrexate, retinoids, and biologics modulating the interleukin-17 (IL-17), IL-23, and tumor necrosis factor α pathways have been used off-label for management.1 Aberrant IL-36 signaling … 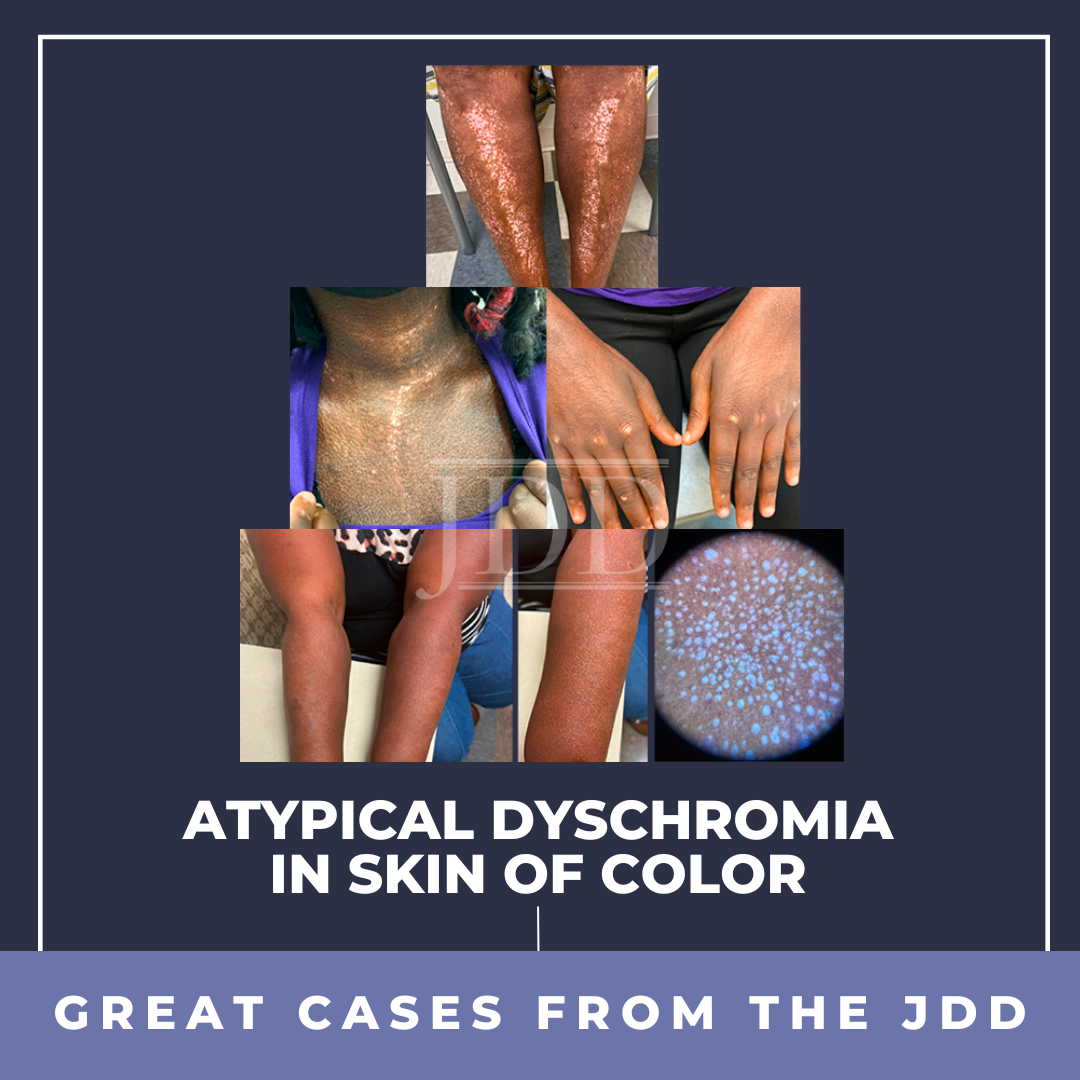 ABSTRACT
Dyschromia is a concern for many patients, especially persons of color. Postinflammatory hypopigmentation and depigmentation can affect all skin types; however, it is more apparent in those with darker skin. Some members of the dermatology community may not comprehensively understand the mechanisms of these reactions and the extent of the psychosocial effect they have on persons of color …
ABSTRACT
Dyschromia is a concern for many patients, especially persons of color. Postinflammatory hypopigmentation and depigmentation can affect all skin types; however, it is more apparent in those with darker skin. Some members of the dermatology community may not comprehensively understand the mechanisms of these reactions and the extent of the psychosocial effect they have on persons of color … 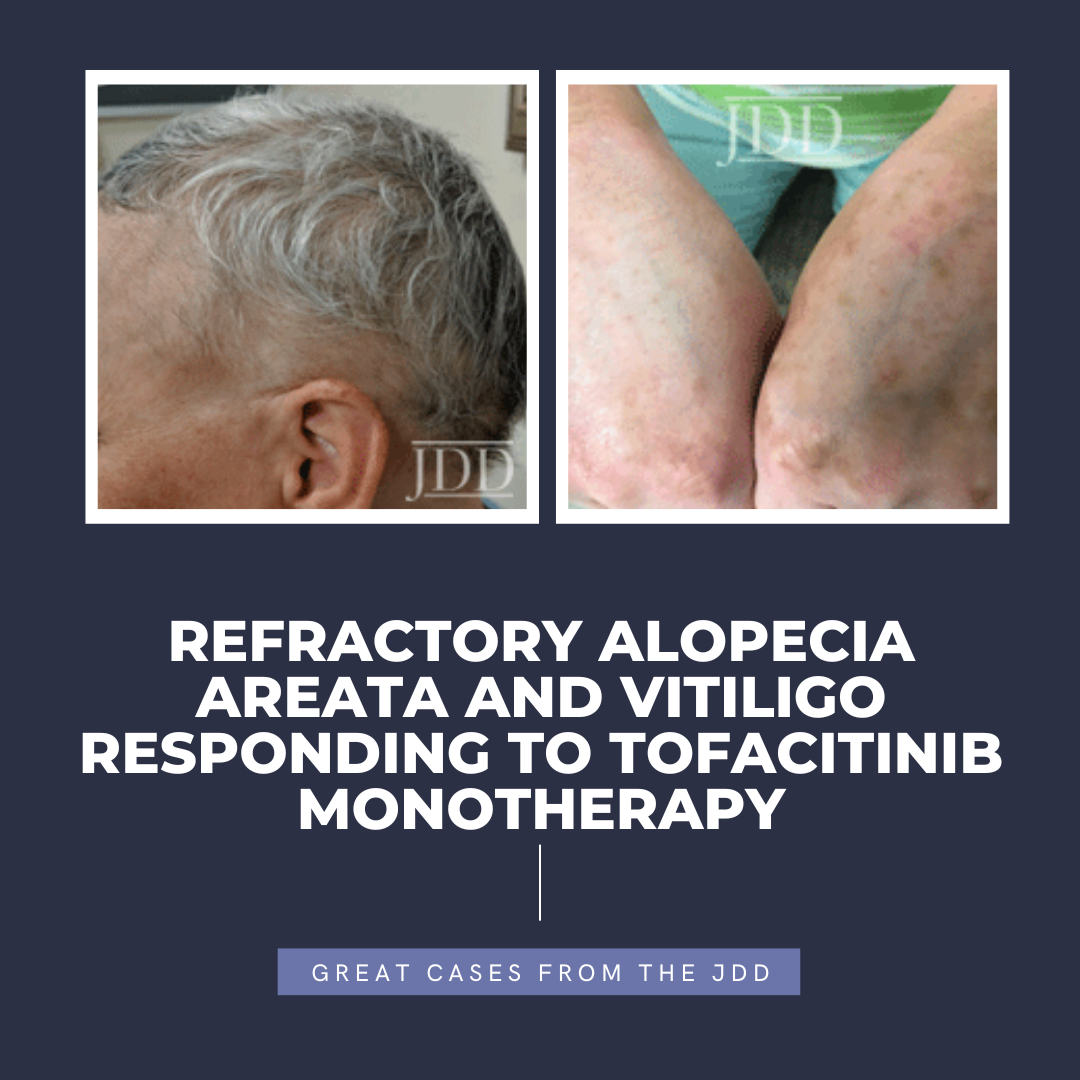 INTRODUCTION
Tofacitinib is a Janus kinase (JAK) 1-3 inhibitor first U.S. Food and Drug Administration (FDA) approved in 2012 for rheumatoid arthritis, with subsequent approval for psoriatic arthritis, ulcerative colitis, polyarticular course juvenile idiopathic arthritis, and ankylosing spondylitis in 2017, 2018, 2020, and 2021, respectively.1,2 In the last several years, oral tofacitinib …
INTRODUCTION
Tofacitinib is a Janus kinase (JAK) 1-3 inhibitor first U.S. Food and Drug Administration (FDA) approved in 2012 for rheumatoid arthritis, with subsequent approval for psoriatic arthritis, ulcerative colitis, polyarticular course juvenile idiopathic arthritis, and ankylosing spondylitis in 2017, 2018, 2020, and 2021, respectively.1,2 In the last several years, oral tofacitinib … 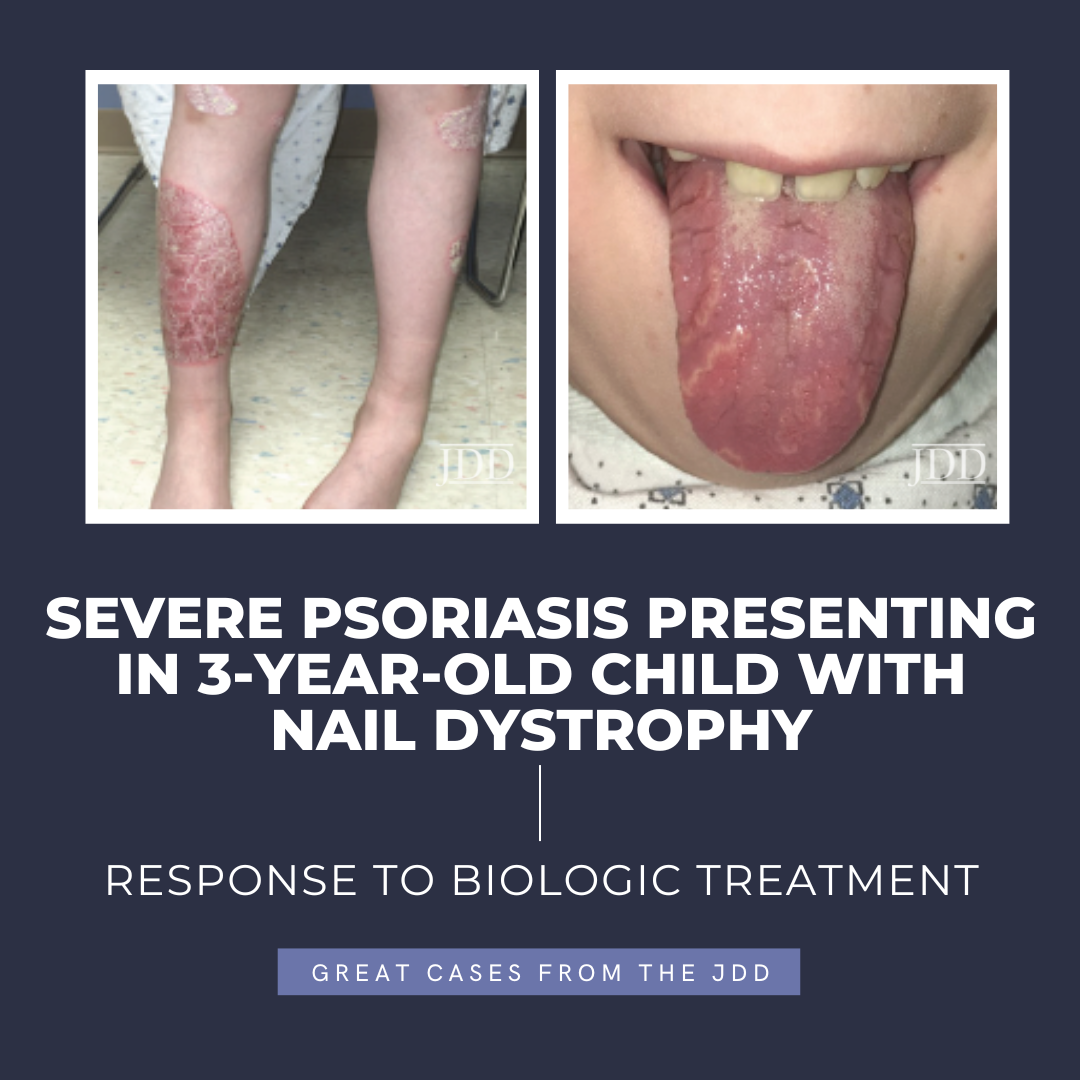 Psoriasis, a chronic inflammatory skin condition, affects about 2% of children. A small subset have isolated nail involvement refractory to topical treatment that can be disabling. The development of targeted biologic agents offers safe, effective options for children with moderate-to-severe skin and nail disease. A few are now Food and Drug Administration (FDA)-approved for children.
INTRODUCTIO …
Psoriasis, a chronic inflammatory skin condition, affects about 2% of children. A small subset have isolated nail involvement refractory to topical treatment that can be disabling. The development of targeted biologic agents offers safe, effective options for children with moderate-to-severe skin and nail disease. A few are now Food and Drug Administration (FDA)-approved for children.
INTRODUCTIO …