Diagnosis and Treatment of Asian-Specific Skin Diseases
 At the 2023 Skin of Color Update Conference, attendees had the exciting opportunity to listen to Dr. George Han, MD, PhD, from Northwell Health in New York, discuss the prevalence and characteristics of certain skin conditions, primarily in Asian populations. His lecture highlighted the differences in the presentation, genetics, and treatment responses among Asians compared to other ethnic groups …
At the 2023 Skin of Color Update Conference, attendees had the exciting opportunity to listen to Dr. George Han, MD, PhD, from Northwell Health in New York, discuss the prevalence and characteristics of certain skin conditions, primarily in Asian populations. His lecture highlighted the differences in the presentation, genetics, and treatment responses among Asians compared to other ethnic groups …
 At the 2023 Skin of Color Update Conference, attendees had the exciting opportunity to listen to Dr. George Han, MD, PhD, from Northwell Health in New York, discuss the prevalence and characteristics of certain skin conditions, primarily in Asian populations. His lecture highlighted the differences in the presentation, genetics, and treatment responses among Asians compared to other ethnic groups …
At the 2023 Skin of Color Update Conference, attendees had the exciting opportunity to listen to Dr. George Han, MD, PhD, from Northwell Health in New York, discuss the prevalence and characteristics of certain skin conditions, primarily in Asian populations. His lecture highlighted the differences in the presentation, genetics, and treatment responses among Asians compared to other ethnic groups … Continue reading "Diagnosis and Treatment of Asian-Specific Skin Diseases"


 With a special focus on the topic of psoriasis, check out highlights from the August issue of the Journal of Drugs in Dermatology (JDD) straight from the JDD Editor’s desk:
Consensus Statements on the Use of Corticosteroid-Containing Topical Medications in Psoriasis delves into the expert guidance provided by the Psoriasis Expert Group (PEG), encompassing nine crucial statements that offer com …
With a special focus on the topic of psoriasis, check out highlights from the August issue of the Journal of Drugs in Dermatology (JDD) straight from the JDD Editor’s desk:
Consensus Statements on the Use of Corticosteroid-Containing Topical Medications in Psoriasis delves into the expert guidance provided by the Psoriasis Expert Group (PEG), encompassing nine crucial statements that offer com … 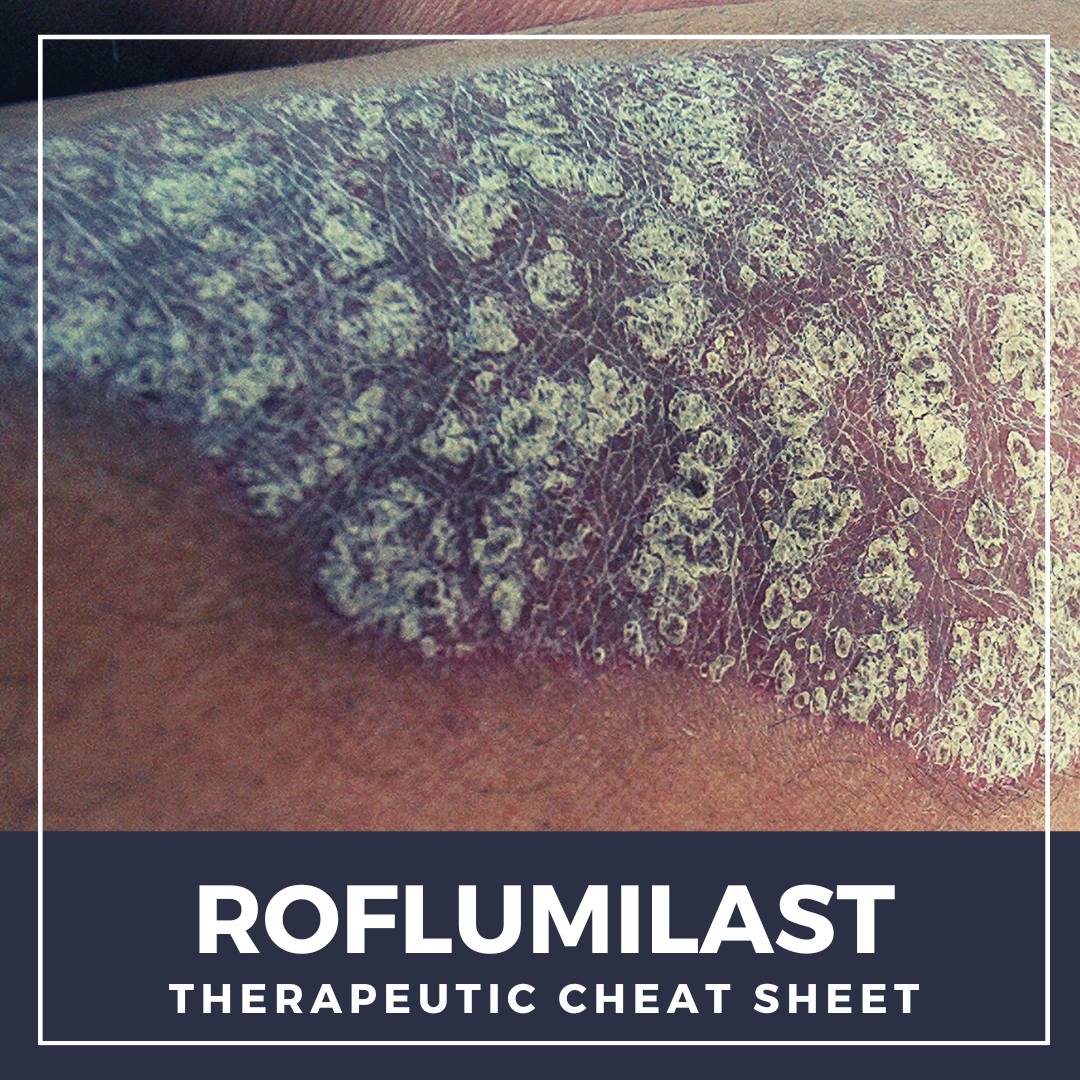 Roflumilast is a non-steroidal topical cream that was FDA approved for the treatment of plaque psoriasis in 2022.1 Roflumilast selectively inhibits phosphodiesterase-4 (PDE-4), which is an enzyme that mediates inflammatory responses and has been shown to play a role in several dermatologic diseases.2 Prior to topical roflumilast, oral PDE-4 inhibitors have been investigated across a wide variety o …
Roflumilast is a non-steroidal topical cream that was FDA approved for the treatment of plaque psoriasis in 2022.1 Roflumilast selectively inhibits phosphodiesterase-4 (PDE-4), which is an enzyme that mediates inflammatory responses and has been shown to play a role in several dermatologic diseases.2 Prior to topical roflumilast, oral PDE-4 inhibitors have been investigated across a wide variety o … 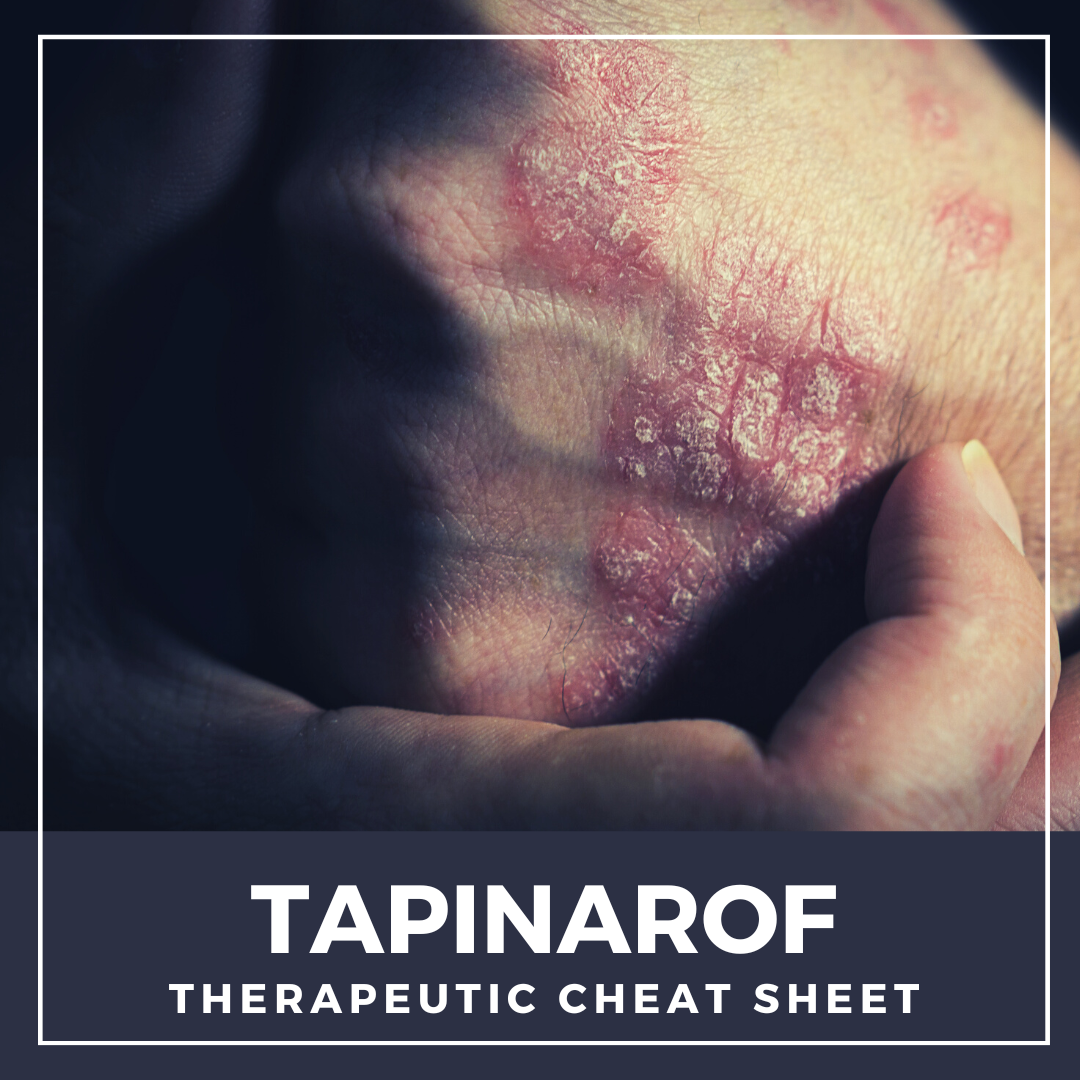 Tapinarof cream is a non-steroidal topical medication that was recently FDA approved in 2022 for the treatment of mild, moderate, or severe plaque psoriasis in adult patients. As biologic medications have continued to grow and become exceedingly present in the treatment of moderate to severe psoriasis, there has been very minimal, if any innovation in the space of topical treatments. Additionally, …
Tapinarof cream is a non-steroidal topical medication that was recently FDA approved in 2022 for the treatment of mild, moderate, or severe plaque psoriasis in adult patients. As biologic medications have continued to grow and become exceedingly present in the treatment of moderate to severe psoriasis, there has been very minimal, if any innovation in the space of topical treatments. Additionally, …  Azelaic acid is a topical therapeutic agent which is FDA approved to treat papules and pustules of mild to moderate rosacea and mild to moderate acne vulgaris. It was first approved by the FDA in 1995 and since its approval, has been used for many off-label conditions including disorders of hyperpigmentation. Its utility in various conditions can be attributed to its anti-microbial, anti-inflamm …
Azelaic acid is a topical therapeutic agent which is FDA approved to treat papules and pustules of mild to moderate rosacea and mild to moderate acne vulgaris. It was first approved by the FDA in 1995 and since its approval, has been used for many off-label conditions including disorders of hyperpigmentation. Its utility in various conditions can be attributed to its anti-microbial, anti-inflamm …