At the 17th Annual ODAC Dermatology, Aesthetic & Surgical Conference held in Orlando, Florida, Dr. Linda Stein Gold, Director of Dermatology Clinical Research and Division Head of Dermatology for the Henry Ford Health System, lectured on new and future treatments for acne. She opened her lecture by describing the traditional view of lesion progression in acne vulgaris, where the microcomedone formation is the first step in the pathogenesis of acne and then factors such as C. acnes proliferation and inflammatory mediators leads to comedones (open and closed) and inflammatory lesions of acne.
To debunk this myth, Dr. Stein Gold shared new research that shows there is subclinical inflammation present before the formation of the microcomedone. In addition, persistent redness, hyperpigmentation, and atrophic scars also show evidence of inflammation on biopsy. This changes the model of how we view the pathogenesis of acne vulgaris. Now it is assumed that there is subclinical (pre-lesional) inflammation that precedes the microcomedone formation and inflammation persists even in the lesions thought to be the sequelae of acne. Furthermore, studies are now showing that there may be more than one type of C. acnes contributing to the onset of acne.
The second part of Dr. Stein Gold’s lecture focused on what’s new and what’s on the horizon for treating acne, beginning with micronized tretinoin lotion. Micronized tretinoin lotion is currently FDA approved to age 9 and is packaged with moisturizing ingredients such as glycerin, collagen, sodium hyaluronate, and purified water to combat dryness. Other than dryness, side effects of the tretinoin formulation include skin burning, irritation, and mild-moderate erythema. This formulation appears to be better tolerated than other tretinoin formulations currently available.
Dr. Stein Gold introduced us to the new research coming out for trifarotene cream, which is a new RARy-selective topical retinoid. Tested in a randomized phase 3 clinical trial, trifarotene was safe and effective at treating moderate acne on both the face and trunk. Patients were able to tolerate the topical cream well and this has been approved for ages 9 and up. This would be a great option for patients suffering from facial and truncal acne.
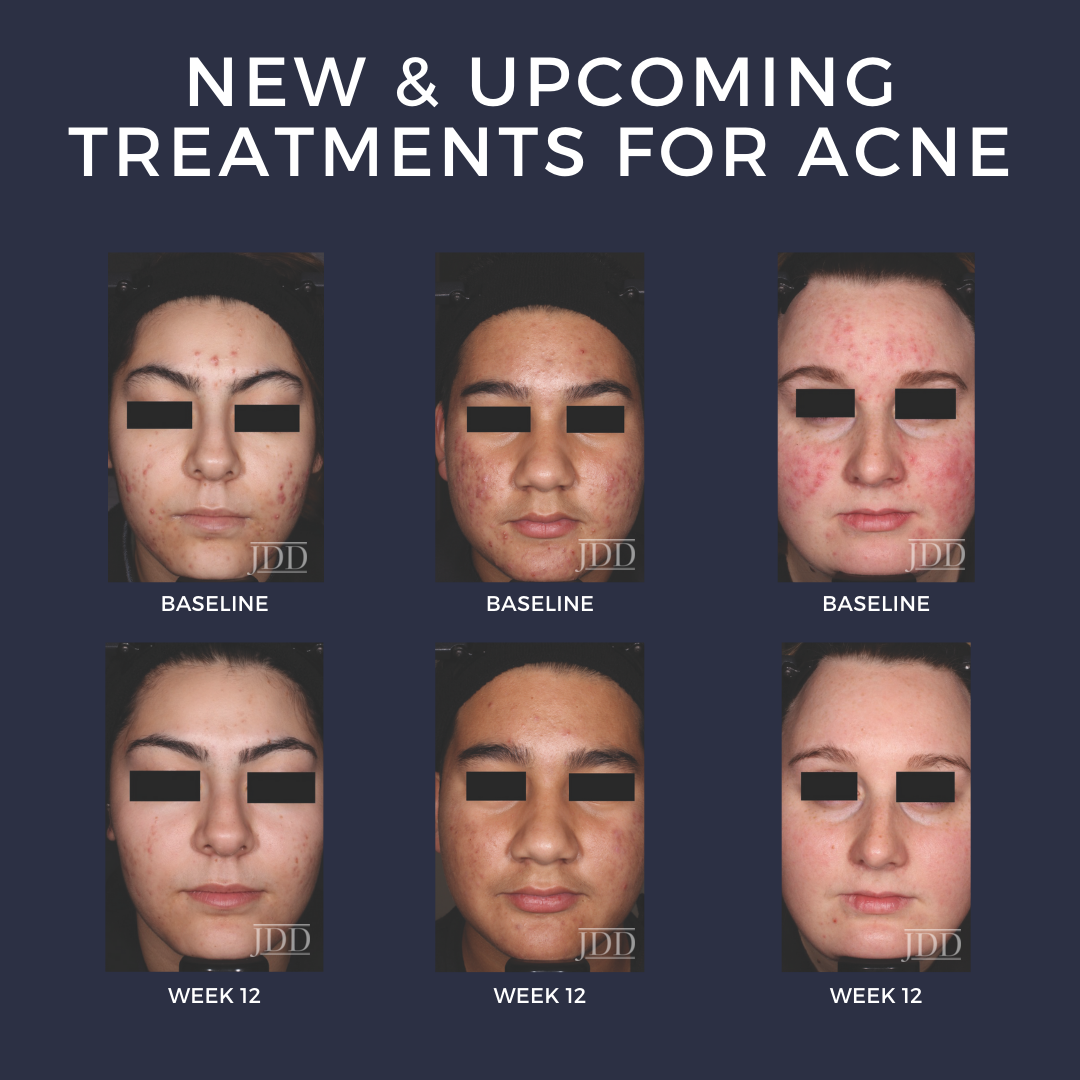
We also learned about tazarotene 0.045% lotion that is formulated for efficient epidermal delivery. Its polymeric emulsion suspension improves penetration of tazarotene on the skin surface. A phase 2 multicenter double-blinded clinical trial evaluated the safety and efficacy of tazarotene 0.045% lotion as compared to tazorac 0.1% cream. All patients in the study were blinded to treatment and had to apply the study drug to the face once a day in the evening for 12 weeks.
Dr. Stein Gold then moved on to discussing retinoids and scarring by posing the question “can retinoids minimize scarring?” Scarring is a significant complication of acne that can often be disfiguring and distressing to the patient. In a multicenter, randomized, investigator-blinded, vehicle-controlled study, subjects with moderate or severe facial acne applied adapalene 0.3%/benzoyl peroxide 2.5% or vehicle daily per half face for 24 weeks. Results show that after 6 months, topical A0.3/BPO2.5 prevented and reduced atrophic scar formation. Scar count increased with vehicle (+ 14.4%) but decreased with A0.3/BPO2.5 (− 15.5%) over 24 weeks.
On antibiotics for acne, Dr. Stein Gold discussed sarecycline, which has improved anti-inflammatory properties and a narrower spectrum of activity than other antibiotics. This drug is dosed on a weight-based dosing, 1.5mg/kg/day and is FDA approved all the way to age 9. In Phase 3 studies, sarecycline significantly decreased the number of inflammatory lesions of acne in patients as compared to placebo. It was also effective at reducing truncal acne on the chest and back. Side effects were rare but included low rates of vaginal yeast infections and dizziness. There were uncommon side effects of sunburn, photosensitivity, and urticaria.
Also, topical minocycline is now FDA approved for acne in the 4% foam. There are a number of pre-clinical trials that were done that showed no evidence of phototoxicity, photoallergy, skin sensitization, or cumulative skin irritation. This drug has completed phase 3 clinical trials. So, what’s different about this drug? This foam formulation has a vehicle that has been shown that when it interacts with sebum, it actually decreases the melting point of sebum. Why do we care about this? When applied to the skin it’s shown to melt sebum a little bit and potentially, we might be able to unclog pores a little bit better allowing enhanced penetration of the drug. When looking at how this drug did in moderate to severe acne (applied once a day), between 15% and 30% of patients got cleared or almost cleared. The drug did well not only for the inflammatory lesions but also for the comedonal lesions. So even though it is a topical minocycline, it works well (maybe because of the vehicle). Dr. Stein Gold explained that although topical acne therapies do not reduce sebum, there is a new topical androgen receptor competitor that has recently completed clinical trials.
Bottom line? It is a really exciting time for the treatment of acne. Dr. Stein Gold’s final pearls were to be aggressive, treat early, and treat for extended periods of time. Even when you see depigmentation and erythema, keep treating to have those anti-inflammatory properties prevent scarring.
Source:
This information was presented by Dr. Linda Stein Gold at the 17th Annual ODAC Dermatology, Aesthetic & Surgical Conference held January 17th-20th, 2020 in Orlando, FL. The above highlights from her lecture were written and compiled by Dr. Nicole A. Negbenebor, PGY-2 dermatology resident at Brown University in Providence, Rhode Island, and one of the 7 residents selected to participate in the Sun Young Dermatology Leader Mentorship Program (a program supported by an educational grant from Sun Pharmaceutical Industries, Inc.).
All images used with permission from the Journal of Drugs in Dermatology.
Did you enjoy this post? Find more on Medical Dermatology here.