Neurotoxins are the mainstay for multiple aesthetic and medical treatments. They are safe and effective treatment for rhytides, but are also temporary, requiring repeat injections about every 3-4 months.1,2 Now, a recently approved formulation of neurotoxin is fulfilling some patient’s desire for a longer lasting effect. Injection of daxibotulinumtoxinA is FDA-approved for treatment of moderate to severe glabellar lines. Studies report its effect lasting as long as 6-9 months in some patients, which some believe is related to a stabilizing excipient peptide used in the product instead of human albumin.1,3 In this Therapeutic Cheat Sheet, we will focus on daxibotulinumtoxinA and its indication for glabellar rhytides.
DaxibotulinumtoxinA Therapeutic Cheat Sheet
Compiled by: Alexis E. Carrington, MD | Reviewed by: Adam Friedman, MD
TRADE NAME
-
- DAXXIFY®
MECHANISM OF ACTION4
-
- DaxibotulinumtoxinA blocks the release of acetylcholine at the neuromuscular junction, resulting in inhibition of muscular movement.
FDA APPROVED FOR4,5
-
- Temporary improvement in the appearance of moderate to severe glabellar wrinkles in adult patients.
OFF-LABEL USES1,3,6-7
-
- Forehead, upper facial and lateral canthus lines
- Upper limb spasticity
- Cervical dystonia
- Plantar fasciitis
DOSING4
-
- Glabellar lines: 8 Units/0.1mL into each of the 5 sites of the glabella, totaling 40 Units. * (Figure 1)
*DaxibotulinumtoxinA comes in 50- and 100-Unit vials. 50 Units are diluted as 0.6 mL and 100 Units are diluted as 1.2 mL resulting in 8 Units.
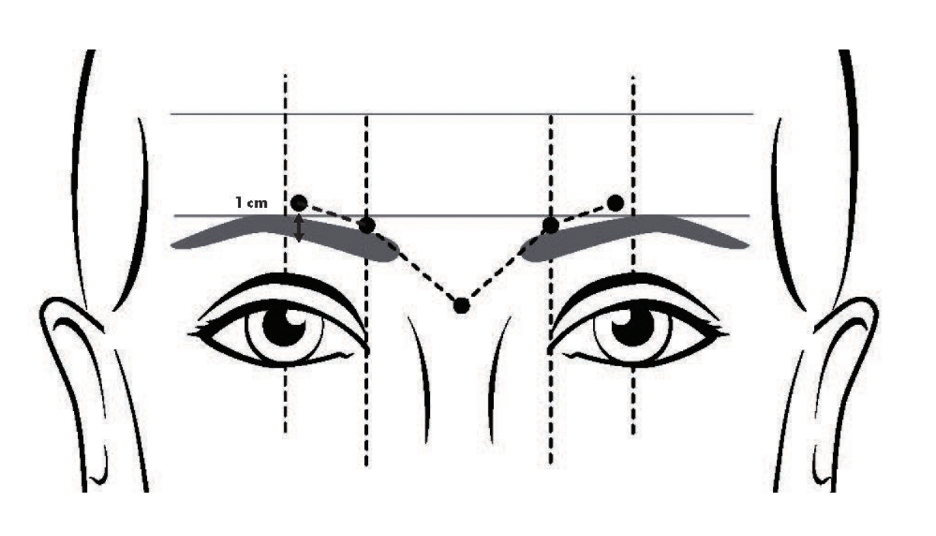 Figure 1. FDA- approved injection sites of daxibotulinumtoxinA for glabellar lines.
Figure 1. FDA- approved injection sites of daxibotulinumtoxinA for glabellar lines.
SIDE EFFECTS4
-
- Headache*
- Eyelid ptosis*
- Facial paresis*
- Injection site pain
- Hemorrhage
- Bruises
- Urticaria
- Dry eye
- Reduced tear production
- Reduced blinking
*Most commonly observed side effects
WARNINGS4
-
- DaxibotulinumtoxinA can spread to areas other than the injection site, resulting in symptoms similar to botulinum toxin hours to weeks after it’s injected. This includes potentially fatal symptoms such as difficulty speaking, swallowing, and breathing.
- Product is not approved for the treatment of spasticity or any locations other than the glabella.
- Rarely, it can cause arrhythmias and myocardial infarction (MI), more common in people with pre-existing cardiovascular conditions.
- Use with caution in patients with compromised respiratory function or dysphagia.
- Concomitant neuromuscular disorder may exacerbate the clinical effects of daxibotulinumtoxinA.
CONTRAINDICATIONS4
-
- Patients with known hypersensitivity to any botulinum toxin, daxibotulinumtoxinA-lanm or any of the components in the DAXXIFY® formulation
- Infection at the injection site
PREGNANCY & BREASTFEEDING4
-
- There is no adequate data on its risk in pregnant women or the risk of major birth defects and miscarriage.
- Animal studies show decreased fetal body weight and skeletal ossification at toxic doses, about 40x the recommended human dose.
- There is no data on the presence of daxibotulinumtoxinA in human or animal milk or its effect on a breastfeeding infant.
MONITORING4
-
- No recommended monitoring guidelines
DaxibotulinumtoxinA is a newly approved effective treatment for glabellar rhytides demonstrating longer lasting results in some scientific studies.8 This and other neurotoxins are demonstrating effectiveness in aesthetic and medical treatments, necessitating providers stay up to date on peer-reviewed research.

References
-
- Salame N, Eber AE, Dover J. DaxibotulinumtoxinA-lanm (Daxxify™): A Comprehensive Overview. Skin Therapy Lett. 2023 Jul;28(4):1-3. PMID: 37440610.
- BOTOX® (onabotulinumtoxin A) [Package Insert]. Irvine, CA: Allergan, Inc.
- Kerscher M, Wanitphakdeedecha R, Trindade de Almeida A, Maas C, Frevert J. IncobotulinumtoxinA: A Highly Purified and Precisely Manufactured Botulinum Neurotoxin Type A. J Drugs Dermatol. 2019 Jan 1;18(1):52-57. PMID: 30681794.
- DAXXIFY™ (DaxibotulinumtoxinA- lanm) [Package Insert]. Newark, CA: Revance Therapeutics, Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761127s000lbl.pdf
- DAXXIFY™ (DaxibotulinumtoxinA- lanm injection) https://daxxify.com/. Accessed 29 Jul. 2023.
- Solish N, Carruthers J, Kaufman J, Rubio RG, Gross TM, Gallagher CJ. Overview of DaxibotulinumtoxinA for Injection: A Novel Formulation of Botulinum Toxin Type A. Drugs. 2021 Dec;81(18):2091-2101. doi: 10.1007/s40265-021-01631-w. Epub 2021 Nov 17. PMID: 34787840; PMCID: PMC8648634.
- Dover JS, Humphrey SD, Lorenc ZP, Shamban A, Gross TM, Rubio RG, Vitarella D. Treatment of Upper Facial Lines With DaxibotulinumtoxinA for Injection: Results From an Open-Label Phase 2 Study. Dermatol Surg. 2023 Jan 1;49(1):60-65. doi: 10.1097/DSS.0000000000003637. Epub 2022 Nov 28. PMID: 36533798; PMCID: PMC9760460.
- Dover JS, Solish N, Gross TM, Gallagher CJ, Brown J. Bridging the Gap: Sustained Treatment Effect of Glabellar Lines With Twice-A-Year Treatment With DaxibotulinumtoxinA. Dermatol Surg. 2023 Jun 22. doi: 10.1097/DSS.0000000000003852. Epub ahead of print. PMID: 37384899.
Did you enjoy this Therapeutic Cheat Sheet? You can find more here.

