Pretibial myxedema (PTM) is a rare complication of Graves’ disease. It is characterized by non-pitting edema with hyperpigmented hyperkeratotic papules and plaques on bilateral lower legs. Effective treatments for patients with PTM are lacking. The etiology of PTM is unknown; however, it may be similar to the mechanism of thyroid-associated ophthalmopathy (TAO). Activated fibroblasts produce inflammatory cytokines and synthesize excessive glycosaminoglycans (GAG) that accumulate in the dermis and subcutaneous tissue. A recent, novel pathway implicates IGF-1 receptor as a mediator in this process. JDD authors Abigail Washington BA et al. present two patients with refractory PTM that improved following treatment with teprotumumab, an IGF-1 receptor inhibitor approved for use in TAO.
INTRODUCTION
Pretibial myxedema (PTM) is a rare manifestation of Graves’ disease and less commonly Hashimoto’s thyroiditis. The progression of Graves’ disease usually begins with thyroid dysfunction, followed by thyroid-associated ophthalmopathy (TAO) and then PTM.1 PTM often presents as non-pitting edema with hyperpigmented hyperkeratotic papules and plaques localized to the pretibial region.
Although there are no FDA approved treatments for PTM, topical and intralesional corticosteroids, rituximab, intravenous immunoglobulin, plasmapheresis, octreotide, and pentoxifylline have been used with variable success.1 Recently teprotumumab, an insulin-like growth factor-1 (IGF-1) receptor inhibitor approved for the treatment for TAO, was reported to improve PTM.2,3,4 We present two cases where the use of teprotumumab improved PTM.2,3
CASES
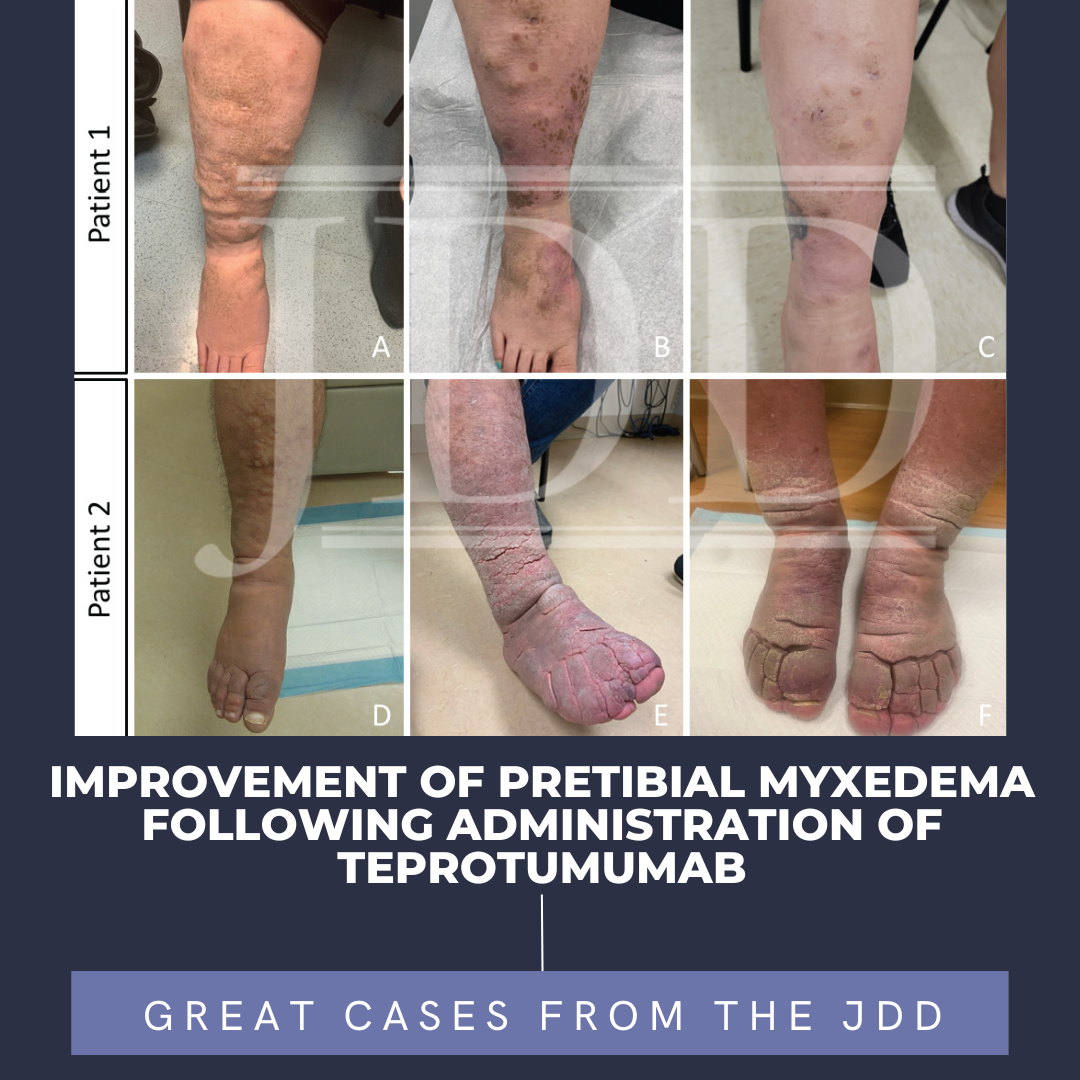
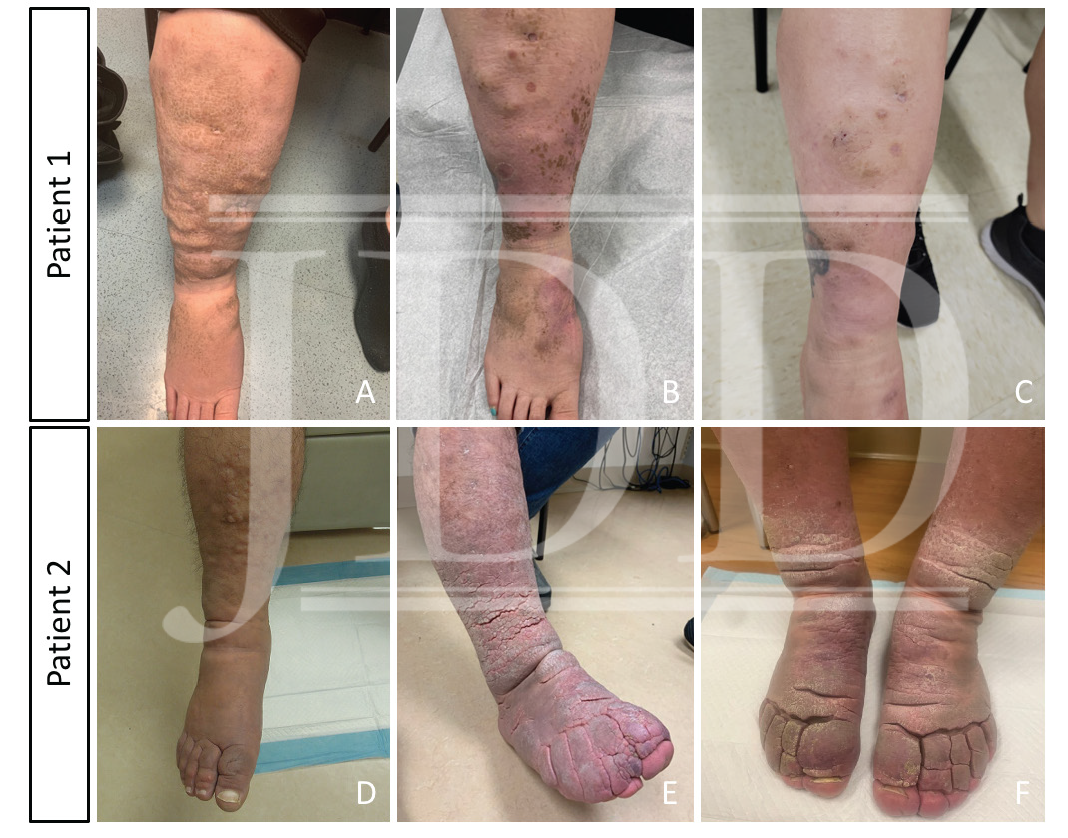
Patient 1 is a 42-year-old female with a history of hyperthyroidism status post radioactive iodine ablation in her teenage years. In 2019, she was diagnosed with Graves’ disease and severe TAO. She was then referred to dermatology in 2020 where firm plaques with plate-like scales were noted on her bilateral lower extremities (Figure 1A), which was biopsied and showed findings consistent with PTM. She was treated with compression, pentoxifylline, and intralesional steroids without improvement. The PTM negatively affected her quality of life making it difficult to ambulate, stand for long periods, and put on shoes. Despite treatment with systemic steroids, radiation therapy to bilateral orbits, and rituximab infusions, her ophthalmopathy and PTM progressed (Figure 1B). Teprotumumab therapy was started for refractory ophthalmopathy in August 2021. She received a shortened course of teprotumumab (five doses) after experiencing intolerable side effects from drug-induced diabetes mellitus. Although she still had remaining plaques, her symptoms of PTM significantly improved by her follow up visit in November 2021 (Figure 1C). Her ophthalmopathy also improved without complete resolution.
Patient 2
Patient 2 is a 55-year-old male with PTM secondary to Graves’ disease. He was first diagnosed with Graves’ disease in 2010 and his thyroid disease was controlled for many years. However, in 2012, he developed edema, nodules, and indurated plaques on the dorsal feet and pretibial lower legs consistent with PTM (Figure 1D). He was treated with compression, pentoxifylline, and intralesional steroids before escalating to hydroxychloroquine, IVIG, rituximab, plasmapheresis, and adalimumab without improvement. The lesions progressed to thickened nodules coalescing into circumferential plaques with superimposed elephantiasis changes (Figure 1E). He also experienced severe TAO (beginning in 2016) that was not responsive to systemic steroids or surgical decompression. In May 2021, he was started on teprotumumab for his TAO and completed the full eight doses without incident. His cutaneous disease improved significantly by his first visit after the infusions in November 2021. He can fit in his normal footwear and tolerate daily activities more comfortably (Figure 1F). His ophthalmopathy also improved following treatment.
DISCUSSION
The pathogenesis of PTM is not completely understood but is thought to be similar to the mechanism of thyroid-associated ophthalmopathy. Patients with PTM have elevated serum levels of thyroid stimulating hormone receptor (TSHR) antibodies, which may interact with IGF-1 to activate fibroblasts.1 IGF-1 is overexpressed in fibroblasts in the pretibial areas and orbital areas, and its activity is necessary for mediating components of TSHR downstream signaling.5 The activated fibroblasts produce inflammatory cytokines and synthesize excessive glycosaminoglycans (GAG).1 GAG accumulation in the dermis and subcutaneous tissue leads to excess fluid buildup and dermal connective tissue expansion, causing the skin manifestations of PTM.1
Teprotumumab is an IGF-1 receptor inhibitor that has been recently FDA approved for treatment of thyroid-associated ophthalmopathy and may have clinical use for the treatment of refractory PTM. Two previous case reports have demonstrated significant improvement of PTM symptoms with the use of teprotumumab.2,3 Adverse events due to teprotumumab included infusion reactions, hyperglycemia, nausea, muscle spasms, diarrhea, and hearing impairment which were almost all grade I or II in severity.4,6
The authors’ patients experienced significant improvement in their PTM symptoms without complete resolution of disease. Teprotumumab is an option for refractory PTM and can significantly increase quality of life for these patients. Further studies are needed to confirm the efficacy and duration of response of teprotumumab in patients with PTM and to explore the optimal dosing regimens.
DISCLOSURES
REFERENCES
3. Petit L, Catinis A, Richard E, Silverberg J. A case of pretibial myxedema treated with teprotumumab. JAAD Case Rep. 2021;16:134-136. doi:10.1016/j. jdcr.2021.08.026
4. Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748-1761. doi:10.1056/ NEJMoa1614949
5. Smith TJ, Janssen JAMJL. Insulin-like growth factor-i receptor and thyroidassociated ophthalmopathy. Endocr Rev. 2019;40(1):236-267. doi:10.1210/ er.2018-00066
6. Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341-352. doi:10.1056/ NEJMoa1910434
SOURCE
Adapted from original article for length and style.