Apremilast Therapeutic Cheat Sheet
 Apremilast (OTEZLA®) is a twice daily oral medication that is FDA approved for adults with plaque psoriasis, psoriatic arthritis and oral ulcers associated with Behçet’s Disease.1 This drug is being extended as an off-label treatment to target inflammation in a number of different conditions. This Therapeutic Cheat Sheet will focus on apremilast and its applications for different dermatologica …
Apremilast (OTEZLA®) is a twice daily oral medication that is FDA approved for adults with plaque psoriasis, psoriatic arthritis and oral ulcers associated with Behçet’s Disease.1 This drug is being extended as an off-label treatment to target inflammation in a number of different conditions. This Therapeutic Cheat Sheet will focus on apremilast and its applications for different dermatologica …
 Apremilast (OTEZLA®) is a twice daily oral medication that is FDA approved for adults with plaque psoriasis, psoriatic arthritis and oral ulcers associated with Behçet’s Disease.1 This drug is being extended as an off-label treatment to target inflammation in a number of different conditions. This Therapeutic Cheat Sheet will focus on apremilast and its applications for different dermatologica …
Apremilast (OTEZLA®) is a twice daily oral medication that is FDA approved for adults with plaque psoriasis, psoriatic arthritis and oral ulcers associated with Behçet’s Disease.1 This drug is being extended as an off-label treatment to target inflammation in a number of different conditions. This Therapeutic Cheat Sheet will focus on apremilast and its applications for different dermatologica … 

 Bustle recently posted an article on tricks to strengthen dry and brittle nails. How should dermatology clinicians counsel their patients who have dry, brittle nails? What nail conditions are sometimes misdiagnosed as dry, brittle nails?
For expert advice, I reached out to Molly Hinshaw, MD, professor of dermatology, section chief of dermatopathology and director of the nail clinic at the U …
Bustle recently posted an article on tricks to strengthen dry and brittle nails. How should dermatology clinicians counsel their patients who have dry, brittle nails? What nail conditions are sometimes misdiagnosed as dry, brittle nails?
For expert advice, I reached out to Molly Hinshaw, MD, professor of dermatology, section chief of dermatopathology and director of the nail clinic at the U …  The February issue of the Journal of Drugs in Dermatology (JDD) includes the perfect blend of original articles, case reports, and brief communications exploring topics such as efficacy and safety of 1% clascoterone cream in patients aged ≥12 years with acne vulgaris, dupilumab’s impact on atopic dermatitis among adolescent and adult patients, development and validation of a photonumeric scale …
The February issue of the Journal of Drugs in Dermatology (JDD) includes the perfect blend of original articles, case reports, and brief communications exploring topics such as efficacy and safety of 1% clascoterone cream in patients aged ≥12 years with acne vulgaris, dupilumab’s impact on atopic dermatitis among adolescent and adult patients, development and validation of a photonumeric scale … 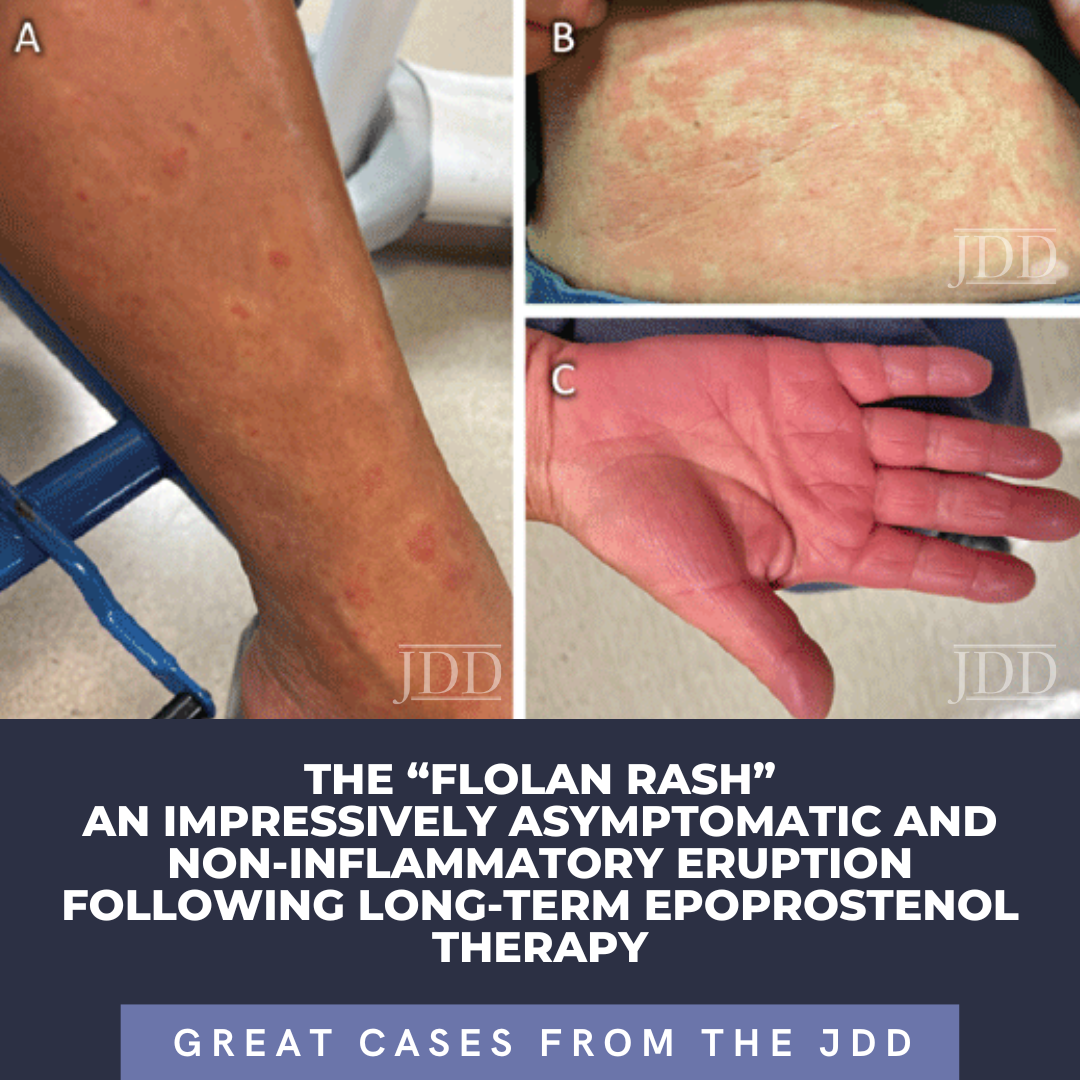 INTRODUCTION
Epoprostenol (Flolan) is a last-resort intravenous (IV) medication for the treatment of severe pulmonary arterial hypertension (PAH). Cutaneous adverse events of Flolan are well-known by pulmonologists, though lacking in dermatologic literature.1 We report an extensive near erythrodermic appearing asymptomatic eruption following long-term use of epoprostenol. This characteristic and …
INTRODUCTION
Epoprostenol (Flolan) is a last-resort intravenous (IV) medication for the treatment of severe pulmonary arterial hypertension (PAH). Cutaneous adverse events of Flolan are well-known by pulmonologists, though lacking in dermatologic literature.1 We report an extensive near erythrodermic appearing asymptomatic eruption following long-term use of epoprostenol. This characteristic and … 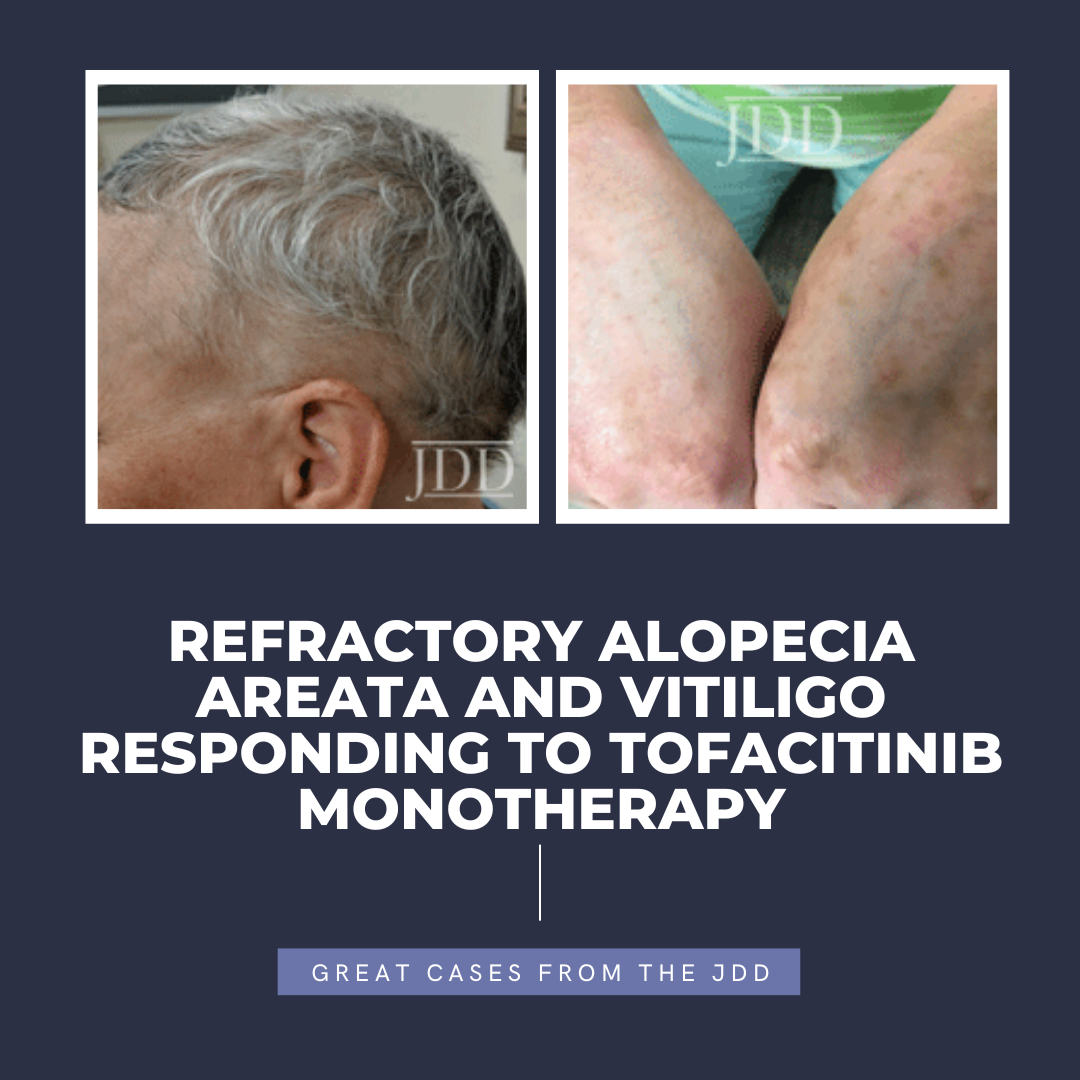 INTRODUCTION
Tofacitinib is a Janus kinase (JAK) 1-3 inhibitor first U.S. Food and Drug Administration (FDA) approved in 2012 for rheumatoid arthritis, with subsequent approval for psoriatic arthritis, ulcerative colitis, polyarticular course juvenile idiopathic arthritis, and ankylosing spondylitis in 2017, 2018, 2020, and 2021, respectively.1,2 In the last several years, oral tofacitinib …
INTRODUCTION
Tofacitinib is a Janus kinase (JAK) 1-3 inhibitor first U.S. Food and Drug Administration (FDA) approved in 2012 for rheumatoid arthritis, with subsequent approval for psoriatic arthritis, ulcerative colitis, polyarticular course juvenile idiopathic arthritis, and ankylosing spondylitis in 2017, 2018, 2020, and 2021, respectively.1,2 In the last several years, oral tofacitinib …