JDD Buzz Series: Oral Minoxidil Shortages After Media Attention
 Media attention about oral minoxidil has impacted access to the hair loss drug in the Washington, D.C. area, according to a study published in the January issue of the Journal of Drugs in Dermatology. Researchers found shortages of the drug in D.C.-area pharmacies more than a year after an article about the treatment appeared in The New York Times.
To find out more about oral minoxidil access, …
Media attention about oral minoxidil has impacted access to the hair loss drug in the Washington, D.C. area, according to a study published in the January issue of the Journal of Drugs in Dermatology. Researchers found shortages of the drug in D.C.-area pharmacies more than a year after an article about the treatment appeared in The New York Times.
To find out more about oral minoxidil access, …
 Media attention about oral minoxidil has impacted access to the hair loss drug in the Washington, D.C. area, according to a study published in the January issue of the Journal of Drugs in Dermatology. Researchers found shortages of the drug in D.C.-area pharmacies more than a year after an article about the treatment appeared in The New York Times.
To find out more about oral minoxidil access, …
Media attention about oral minoxidil has impacted access to the hair loss drug in the Washington, D.C. area, according to a study published in the January issue of the Journal of Drugs in Dermatology. Researchers found shortages of the drug in D.C.-area pharmacies more than a year after an article about the treatment appeared in The New York Times.
To find out more about oral minoxidil access, … Continue reading "JDD Buzz Series: Oral Minoxidil Shortages After Media Attention"


 Intravenous immune globulin (IVIG) is a concentrate of pooled immunoglobulins derived from plasma donors. Its unique mechanism of action expands the utility of the medication to a variety of conditions. We continue our series, Therapeutic Cheat Sheet, with a closer look at IVIG, which is FDA-approved for the treatment of dermatologic conditions including dermatomyositis, Kawasaki disease, ITP, and …
Intravenous immune globulin (IVIG) is a concentrate of pooled immunoglobulins derived from plasma donors. Its unique mechanism of action expands the utility of the medication to a variety of conditions. We continue our series, Therapeutic Cheat Sheet, with a closer look at IVIG, which is FDA-approved for the treatment of dermatologic conditions including dermatomyositis, Kawasaki disease, ITP, and … 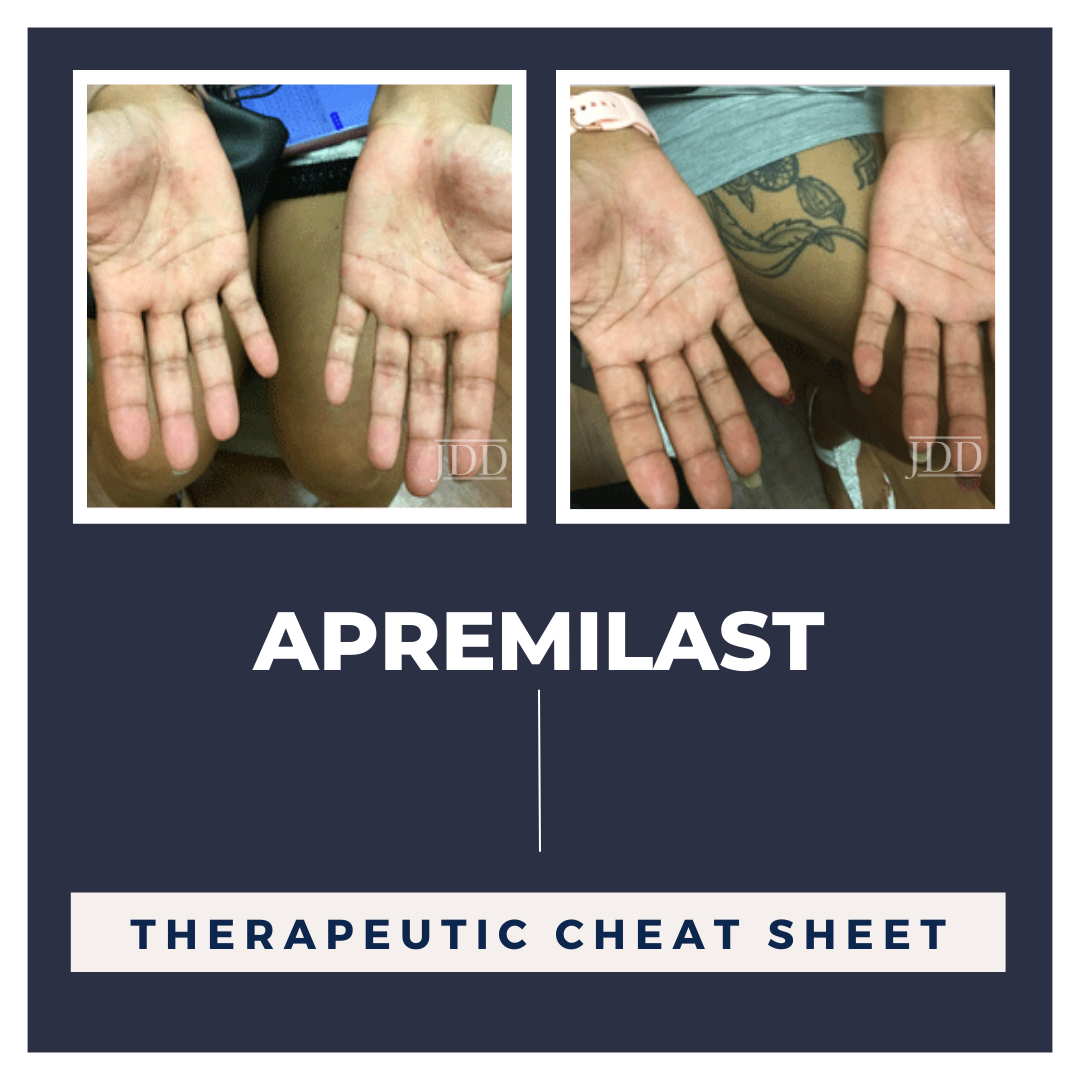 Apremilast (OTEZLA®) is a twice daily oral medication that is FDA approved for adults with plaque psoriasis, psoriatic arthritis and oral ulcers associated with Behçet’s Disease.1 This drug is being extended as an off-label treatment to target inflammation in a number of different conditions. This Therapeutic Cheat Sheet will focus on apremilast and its applications for different dermatologica …
Apremilast (OTEZLA®) is a twice daily oral medication that is FDA approved for adults with plaque psoriasis, psoriatic arthritis and oral ulcers associated with Behçet’s Disease.1 This drug is being extended as an off-label treatment to target inflammation in a number of different conditions. This Therapeutic Cheat Sheet will focus on apremilast and its applications for different dermatologica …  Next Steps in Derm and the Journal of Drugs in Dermatology, in partnership with the Dermatology Education Foundation (DEF) and Physicians Resources, interviewed Dr. Adam Friedman, professor and chair of dermatology at GW School of Medicine and Health Sciences, on life lessons from his residency and career. Dr. Friedman provides his tips for remaining calm during the craziest of office visits. …
Next Steps in Derm and the Journal of Drugs in Dermatology, in partnership with the Dermatology Education Foundation (DEF) and Physicians Resources, interviewed Dr. Adam Friedman, professor and chair of dermatology at GW School of Medicine and Health Sciences, on life lessons from his residency and career. Dr. Friedman provides his tips for remaining calm during the craziest of office visits. … 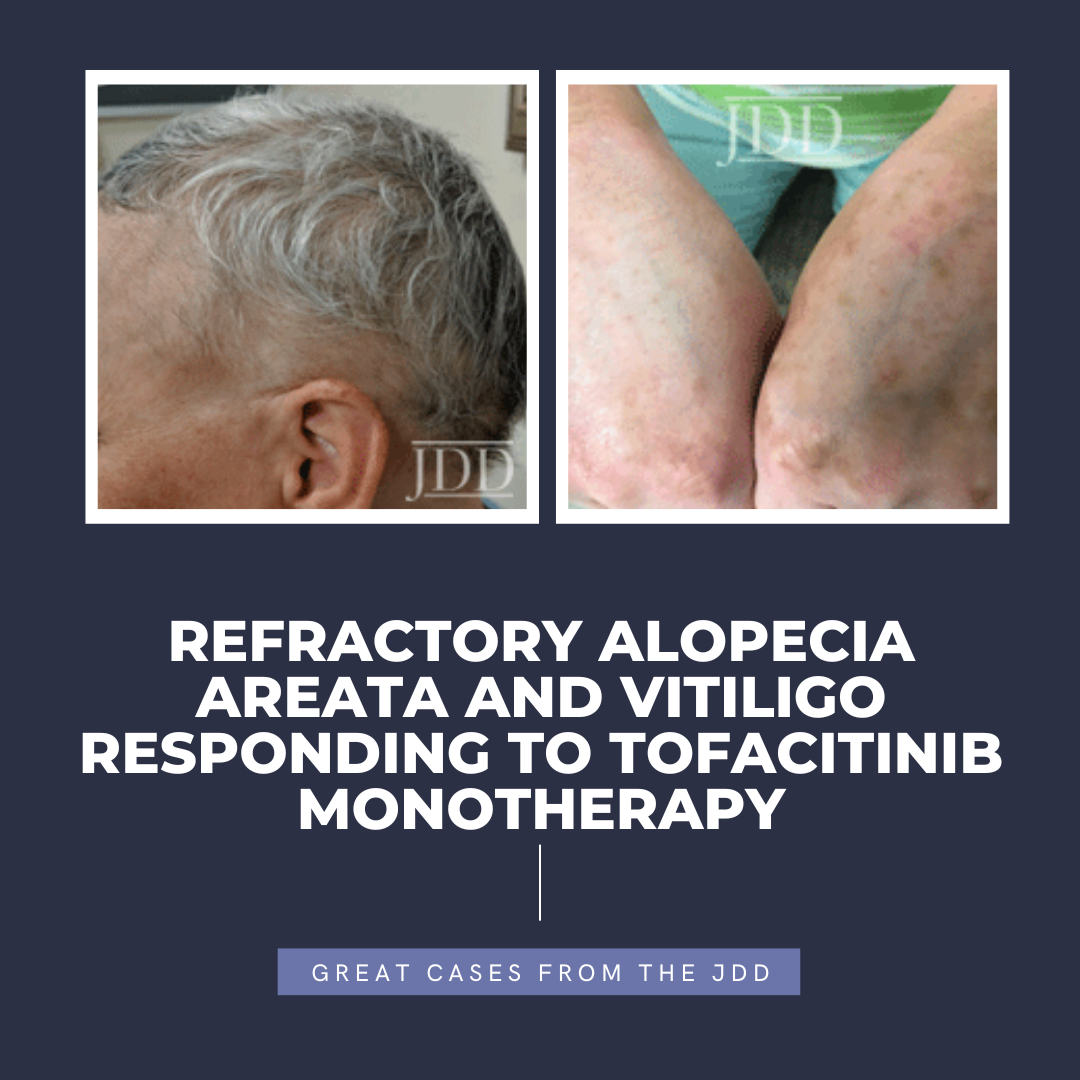 INTRODUCTION
Tofacitinib is a Janus kinase (JAK) 1-3 inhibitor first U.S. Food and Drug Administration (FDA) approved in 2012 for rheumatoid arthritis, with subsequent approval for psoriatic arthritis, ulcerative colitis, polyarticular course juvenile idiopathic arthritis, and ankylosing spondylitis in 2017, 2018, 2020, and 2021, respectively.1,2 In the last several years, oral tofacitinib …
INTRODUCTION
Tofacitinib is a Janus kinase (JAK) 1-3 inhibitor first U.S. Food and Drug Administration (FDA) approved in 2012 for rheumatoid arthritis, with subsequent approval for psoriatic arthritis, ulcerative colitis, polyarticular course juvenile idiopathic arthritis, and ankylosing spondylitis in 2017, 2018, 2020, and 2021, respectively.1,2 In the last several years, oral tofacitinib …