Eruptive Squamous Cell Carcinomas Following Treatment With Fludarabine
 JDD authors Mihir Shah MD, Jenna Wald MD, and C. William Hanke MD MPH present a case of a patient with eruptive squamous cell carcinomas following treatment with Fludarabine to highlight not only the risk of cSCC in CLL patients and the increased risk for atypical cutaneous malignancies after treatment with systemic therapies such as fludarabine, but also to discuss treatment options for this …
JDD authors Mihir Shah MD, Jenna Wald MD, and C. William Hanke MD MPH present a case of a patient with eruptive squamous cell carcinomas following treatment with Fludarabine to highlight not only the risk of cSCC in CLL patients and the increased risk for atypical cutaneous malignancies after treatment with systemic therapies such as fludarabine, but also to discuss treatment options for this …
 JDD authors Mihir Shah MD, Jenna Wald MD, and C. William Hanke MD MPH present a case of a patient with eruptive squamous cell carcinomas following treatment with Fludarabine to highlight not only the risk of cSCC in CLL patients and the increased risk for atypical cutaneous malignancies after treatment with systemic therapies such as fludarabine, but also to discuss treatment options for this …
JDD authors Mihir Shah MD, Jenna Wald MD, and C. William Hanke MD MPH present a case of a patient with eruptive squamous cell carcinomas following treatment with Fludarabine to highlight not only the risk of cSCC in CLL patients and the increased risk for atypical cutaneous malignancies after treatment with systemic therapies such as fludarabine, but also to discuss treatment options for this … Continue reading "Eruptive Squamous Cell Carcinomas Following Treatment With Fludarabine"


 Perifolliculitis capitis abscedens et suffodiens or dissecting cellulitis (DC) is a rare and chronic disease with a predilection for the occipital, vertex, and parietal scalp. DC is characterized by multinodular lesions with purulent drainage and sinus tract formation. It is classically seen in middle-aged males of African descent. The etiology of the disease is unknown; however, leading theories …
Perifolliculitis capitis abscedens et suffodiens or dissecting cellulitis (DC) is a rare and chronic disease with a predilection for the occipital, vertex, and parietal scalp. DC is characterized by multinodular lesions with purulent drainage and sinus tract formation. It is classically seen in middle-aged males of African descent. The etiology of the disease is unknown; however, leading theories … 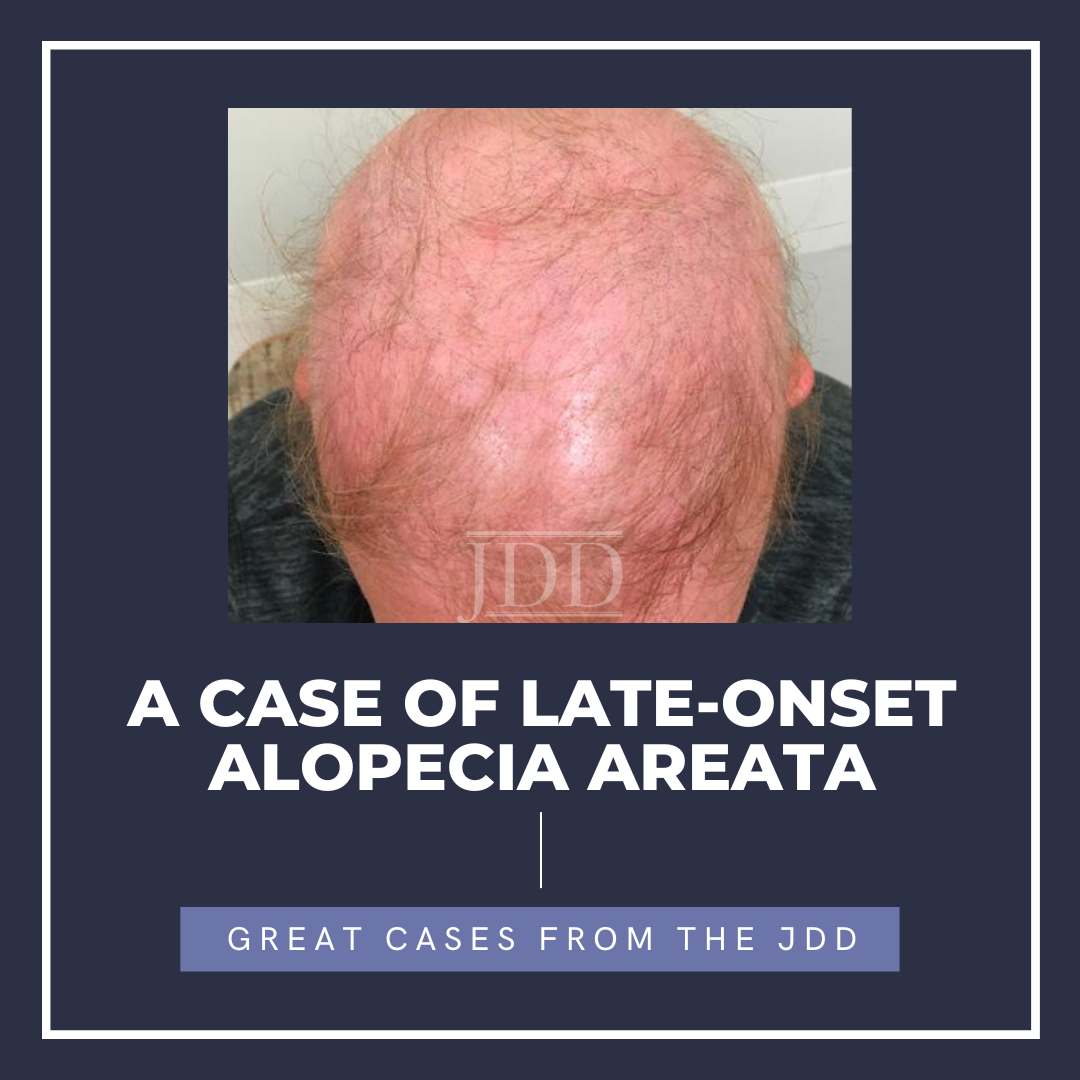 INTRODUCTION
Alopecia areata (AA) is a condition characterized by nonscarring hair loss. Cases of alopecia areata are most commonly seen in patients under age 30 and are frequently idiopathic. In this report, we discuss a woman in her 50s who developed AA shortly after receiving the Tdap vaccine and after one year of guselkumab therapy.
CASE
A woman in her 50s with history of psor …
INTRODUCTION
Alopecia areata (AA) is a condition characterized by nonscarring hair loss. Cases of alopecia areata are most commonly seen in patients under age 30 and are frequently idiopathic. In this report, we discuss a woman in her 50s who developed AA shortly after receiving the Tdap vaccine and after one year of guselkumab therapy.
CASE
A woman in her 50s with history of psor … 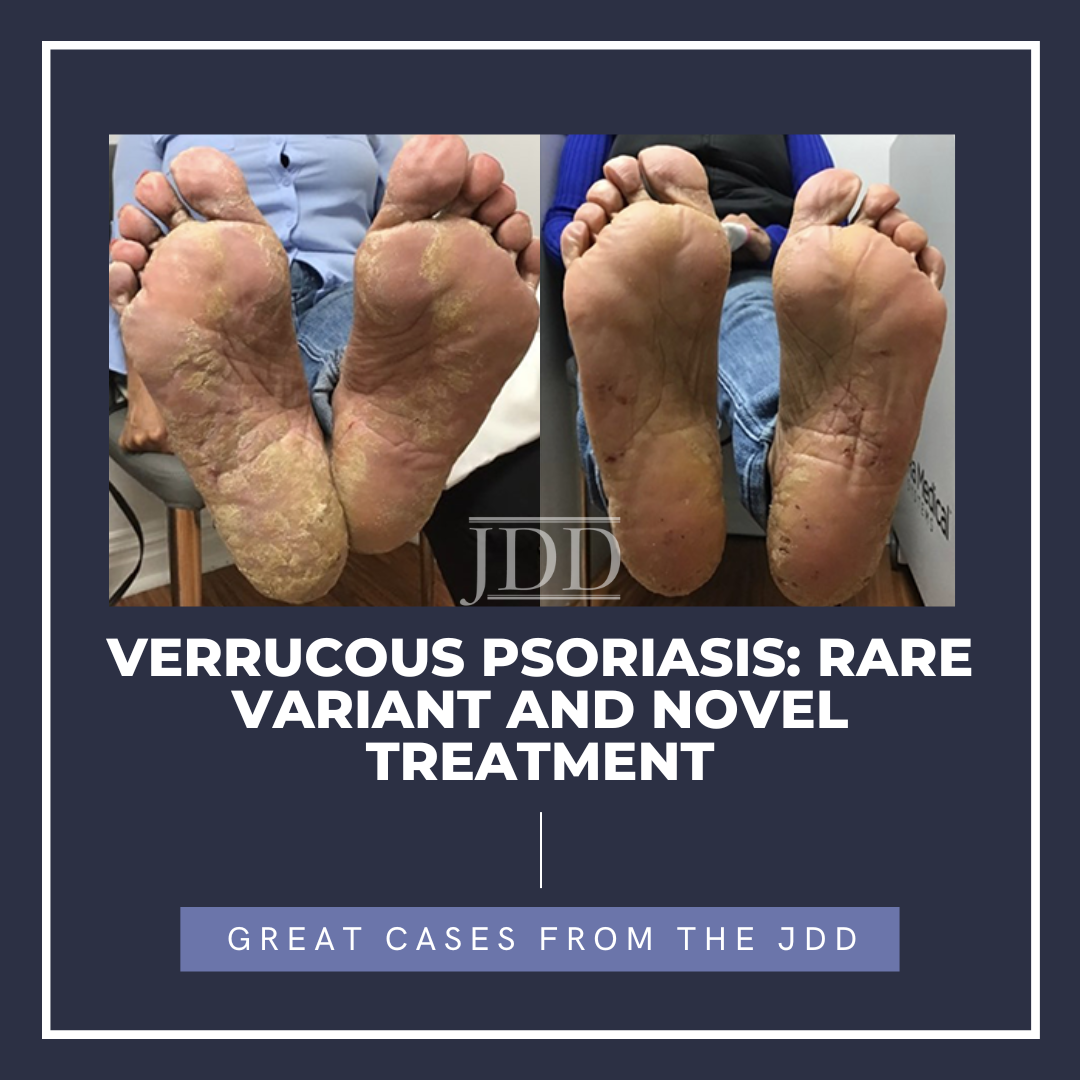 JDD authors Dimitra Xenopoulou MS, Christopher Pochat MS, and Evelyn Greco DO present the findings of a successful treatment for verrucous psoriasis.
CASE
A 64-year-old female presented to the outpatient clinic for the evaluation of flaking and itchy lesions on her bilateral hands and feet that were present for several months and caused difficulty with activities of daily living. Inconsist …
JDD authors Dimitra Xenopoulou MS, Christopher Pochat MS, and Evelyn Greco DO present the findings of a successful treatment for verrucous psoriasis.
CASE
A 64-year-old female presented to the outpatient clinic for the evaluation of flaking and itchy lesions on her bilateral hands and feet that were present for several months and caused difficulty with activities of daily living. Inconsist … 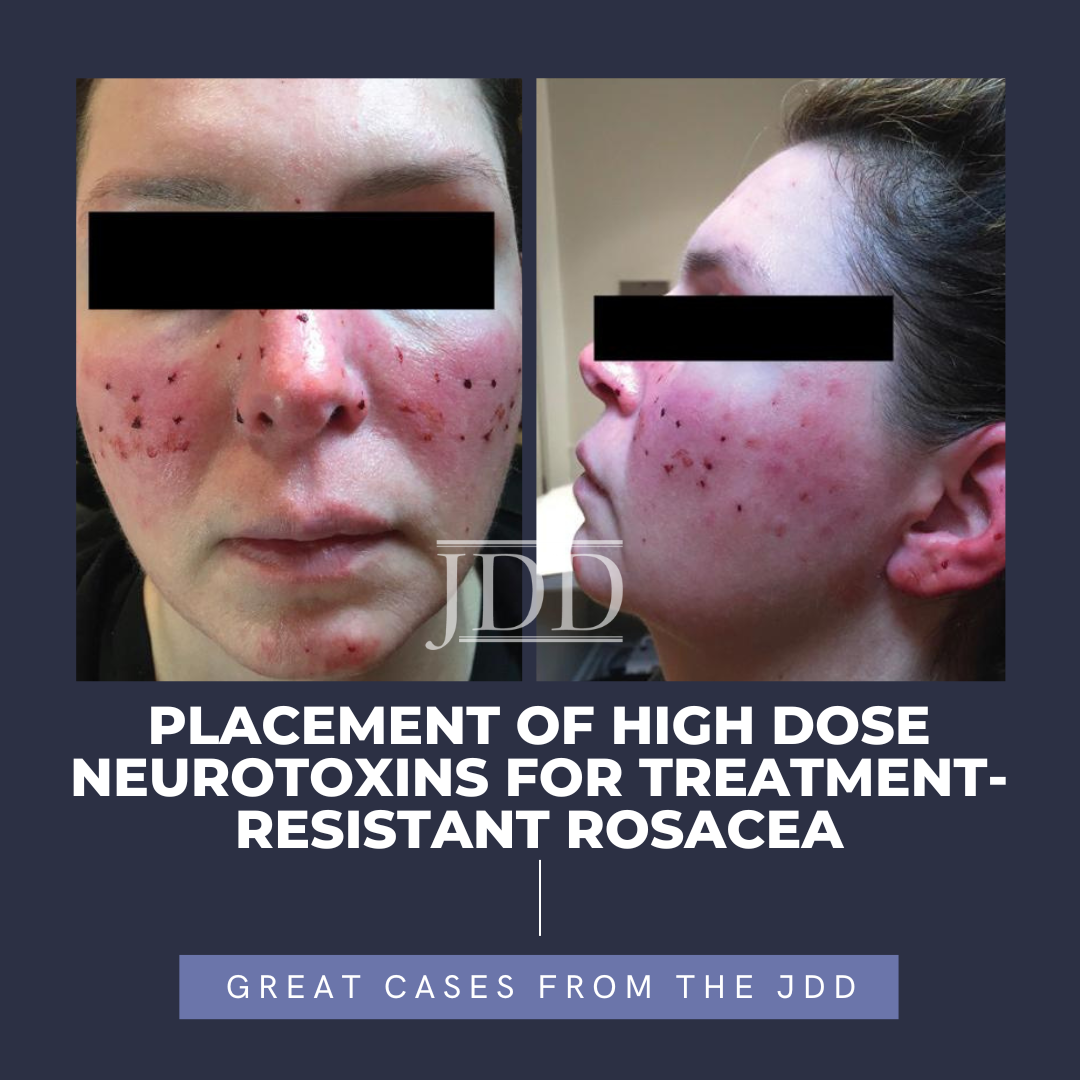 Rosacea is a chronic inflammatory skin condition characterized by facial flushing, erythema, telangiectasias, and papulopustular lesions. Treatment for rosacea includes limiting inciting factors and reducing inflammation with topical and oral therapies. Traditional therapies primarily focus on the papulopustular or background erythematotelangiectatic component of rosacea, leaving symptoms of flush …
Rosacea is a chronic inflammatory skin condition characterized by facial flushing, erythema, telangiectasias, and papulopustular lesions. Treatment for rosacea includes limiting inciting factors and reducing inflammation with topical and oral therapies. Traditional therapies primarily focus on the papulopustular or background erythematotelangiectatic component of rosacea, leaving symptoms of flush …