Admittedly, it took me a while to get over the fear of an artificial intelligence (AI) “apocalypse”, which likely developed after my older brother forced me to repeatedly watch “The Terminator” at the tender age of seven. Through an extensive dive into the literature and numerous lectures by Dr. Vishal A. Patel, I’ve since realized the applicability and patient benefit of AI in dermatology is far from apocalyptic. This is a can’t miss article highlighting the questions that I personally and likely numerous other in-training, young, or experienced dermatologists have regarding the application of AI to dermatology.
-Michael J. Visconti, DO
Despite melanoma being the third most common skin cancer in the United States, we currently practice in a world where melanoma-related biologic behavior (aggression, lymph node spread, metastasis) is not readily predicted. At a cost to both patients and the health system, pigmented lesions are frequently biopsied with only a small portion read out as melanoma or demonstrating a degree of dysplasia requiring further action. Moreover, it remains uncertain which thin melanomas will go on to metastasize and/or recur after a given time period, in addition to which thicker melanomas will behave less aggressively. Lastly, despite having the ability to detect a mutant melanoma-causing gene (like BRAF) and provide pinpoint therapy, we have only been able to treat a marginal number of melanomas effectively due to unforeseen factors complicating prognosis and treatment. In an ideal world of melanoma management, we would be able to tell patients pre-biopsy if a lesion has features suggestive of aggressive behavior, provide a risk of recurrence at the time of diagnosis, develop a personalized, tumor-specific therapeutic regimen, and have the difficult conversations about long-term risk of metastasis and death supplemented with the support of actionable data.
Enter the realm of artificial intelligence.
QUESTION #1: What is the goal of AI in the classification and management of melanoma?
As stated above, AI technology aims to supplement (not replace) physicians in melanoma-related diagnostic and prognostic scenarios and thus help to provide robust, actionable evidence in the care and management of melanocytic lesions. Importantly, AI is not aiming to replace the clinical acumen of physicians. Although frequently thought of as a “substitute” or “threat” for dermatologists and pathologists (and sparking trepidation to its acceptance), AI is rather a tool to supplement the acumen of trained physicians and improve the management of melanoma.
QUESTION #2: What exactly is AI? How does AI work?
To further speak the language of AI, there are numerous definitions that need to be learned. Hang in there; we plan to highlight only a few critical terms to aid your overall grasp of this complicated topic and provide a conversational understanding.
Our first term is, of course, AI. As a whole, AI merely refers to the use of machines to simulate human cognition. We implore AI with the hope of solving the tough clinical questions we as physicians face. In melanoma management, this specifically can assist with the management of those melanomas landing in the “grey zone” of management, where our current data falls short of providing concrete, actionable items. Before exploring further, it’s imperative to remember the basis of simulating human cognition is adaptive advanced statistical modeling. Input data points (an address or location, for example) is fed into the statistical model, and a single or multiple output data point(s) (directions) are generated.
In order to explain the subclassifications of AI, we’ll use an analogy applicable to all of us. Imagine AI as a smartphone. Your smartphone has a brain, memory, and applications to supplement its use. The applications you install and use (i.e., a navigation application) rely on the smartphone’s brain and memory to provide actionable items (i.e., turn right on Main Street).
If AI as a whole is the smartphone, then machine learning (our next term) can be thought of as a navigation application that is used to get to work each day. You provide input data points (destination, time of departure, planned pit-stops), and an output data point is generated (overall distance, estimated time of arrival). Because the machine learning navigation application is operating within a predetermined set of parameters (i.e., provide overall distance and estimated time of arrival based on destination, time of departure), the output data produced by machine learning is limited to only that parameter. Additionally, you can imagine that creating and implementing these parameters was incredibly time-consuming for the software developers who created the application.
On the other hand, deep learning is not bound by a predetermined set of parameters. Deep learning relies on artificial neural networks (ANNs) or convolutional neural networks (CNNs) to digest a wider breadth of input data points and thus provide a plethora of possible output data points. This would be analogous to a navigation application that, after inputting your destination and time of departure, not only tells you the overall distance and time of arrival, but also provides information on traffic jams, car accidents, speed checks, suggests the best route, and decides whether or not now is the best time to leave or if you should wait. Furthermore, since there were no predetermined parameters, a large amount of time was saved in creating the application.
QUESTION #3: What are a few currently developed clinical applications?
Today, two available applications of AI in dermatology include convolutional neural networks (CNNs) and gene expression profiling (GEP).
Keeping in line with the smartphone analogy, CNNs can be thought of as a vastly more advanced facial recognition software on your smartphone. An input data point is presented (an image of a face), and an output data point is generated (face recognized), translating into the smartphone being unlocked. If we imagine a vast expansion of this facial recognition software to recognize and remember every input data point (every face) it encounters and the corresponding output data point that was generated (recognized/not recognized, keep smartphone locked) to develop a more experienced statistical model, we would be close to realizing the application of CNNs in the characterization and subsequent management of pigmented lesions.
Pigmented lesions are presented in macroscopic, microscopic, or dermatoscopic images (input data points) to the advanced statistical model, followed by pixel-level information extraction. Without limitation from preset parameters, the CNN is able to group the presented image based on past images the CNN has encountered into a category of benign or malignant. This exposure over time can allow the statistical model to self-create a hierarchy of lesion classification without an input of parameters from developers.
Current CNN-based models in dermatology have been shown to perform as well as one standard deviation above dermatologists. Regardless of experience level, CNNs have been shown to benefit the classification of pigmented lesions by dermatologists.
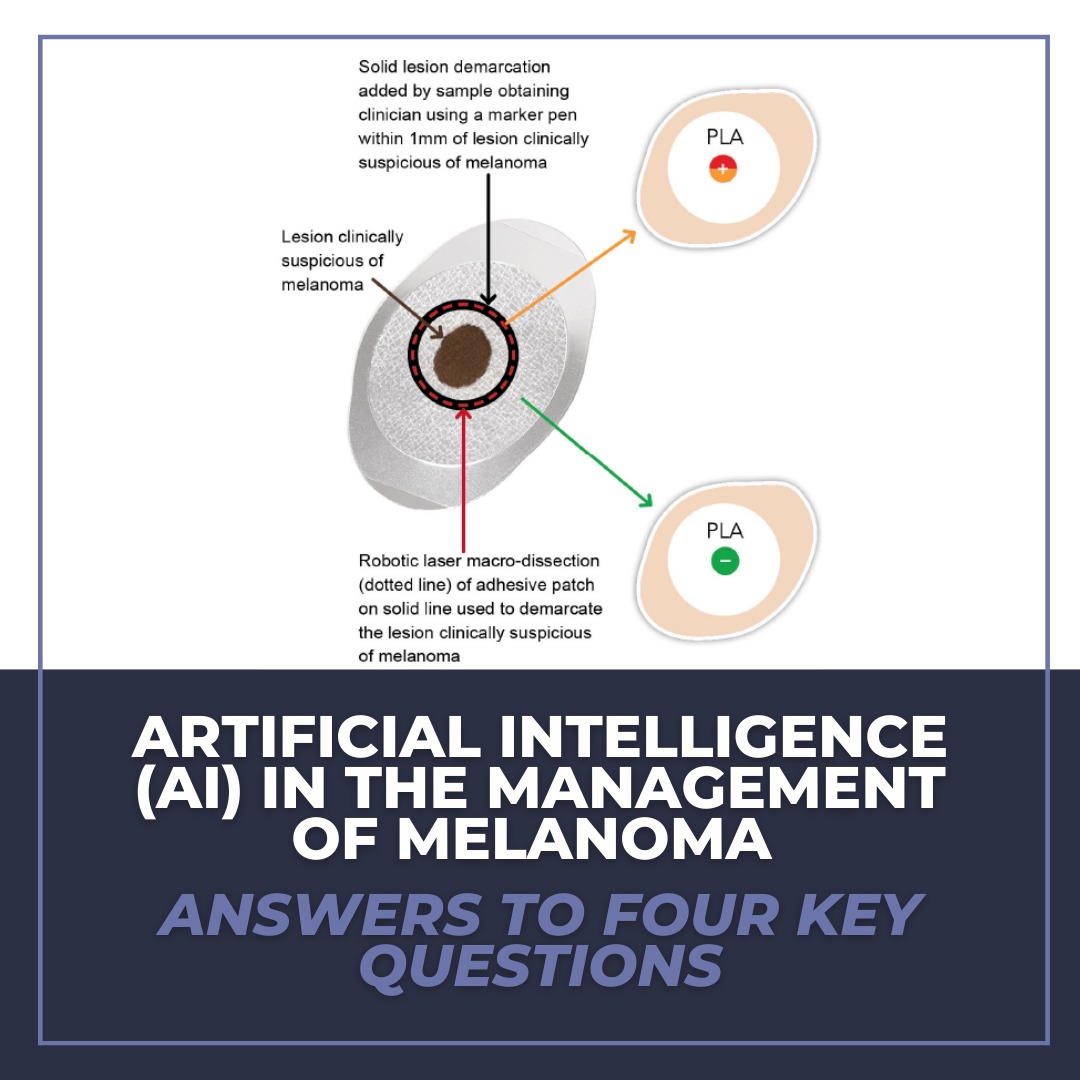
Another specific modality of AI is gene expression profiling (GEP). Examples include Castle Biosciences, Inc. DecisionDx-Melanoma and DermTech’s Pigmented Lesion Assay. In both of these products, GEP relies on input data points (frequencies and intensities of gene activity obtained via molecular probes) for numerous genes to provide output data points (gene expression profile) for pigmented cutaneous lesions. The output in this situation (gene expression profile) could be used to determine lesion behavior. After the long-term accumulation of input and output data points with correlated management decisions, GEP has the potential to provide potentially actionable items in melanoma management pre- and post-biopsy.
An intriguing recent discovery in GEP-testing analyzes the RNA ratio of genes in a population of keratinocytes and melanocytes. By doing so, it would be able to output a ratio of these two cell populations. Instead of solely commenting on “thickness” of a lesion, it could analyze cellular “density” of a lesion as well to further classify tumors. Melanocyte density, as opposed to solely relying on thickness, may allow us to better predict thin stage I melanomas that are incorrectly classified as having a low rate of recurrence/metastasis in addition to thicker stage II melanomas are incorrectly classified as having a high risk of recurrence/metastasis.
One important point is that the output data points cannot be used interchangeably. For example, if a model is developed to predict the risk of nodal metastases of a tumor based on it’s gene expression profile, this will not automatically translate into a predictor of overall mortality or recurrence of the tumor. In other words, you can’t use the Costco shopping application on your smartphone when you’re shopping at Target despite both applications working off of similar algorithms/statistical models.
QUESTION #4: When will we fully adopt AI into our specialty? What is next?
Allow us to nerd out one last time to provide a framework to answer this important question. Machine learning (to mimic human cognition) is only attainable with advanced statistical modeling. With advanced statistical modeling, we can provide an input (i.e., morphological, dermoscopic, histopathological, and genetic features) to generate an output (i.e., tumor behavior, risk of nodal invasion, therapeutic response). Over time, through rigorous data collecting and vetting, we may be able to generate actionable items for clinical practice of melanocytic lesions. The more advanced the statistical model becomes, the higher degree of predictability, and reliability. While utilizing deep learning technology allows for the bypass of generating preset parameters, it still relies on an extensive exposure and learning process via standardized, vetted input data points called “known truths”.
It may be evident from this description that the missing, rate-limiting variables preventing adoption of AI into clinical practice are input data (and subsequent output data generation) and time. Both input and output data points must be vetted for accuracy and reproducibility to allow the machine to learn in a safe environment. Unfortunately, there is no literature to support a universally required amount of data input before making AI tools clinically reliable in melanoma management (or any application for that matter). Furthermore, it’s important to remember the length of time required to complete our current system of prognostication through the MSLT-1 and MSLT-2 trials, which took nearly ten years.
It’s difficult to predict when new guidelines will come out regarding AI implementation in dermatology. If there is any major takeaway from this article, it’s that histology and sentinel lymph node biopsy remain the iron-clad methods for prognostication in melanoma as we are not at a place currently where we can solely rely on AI technology.
CONCLUSION
In an ideal world of pigmented lesion management, several scenarios exist. We would be able to tell patients at the time of their cutaneous malignancy diagnosis what their specific risk of recurrence is. We would be able to have that conversation with patients about their long-term risk of metastasis or death. We could predict which thin melanomas in a young patient will behave aggressively and warrant further work-up or more aggressive therapeutic management. With those goals stated, we should acknowledge our current system is not quite able to reliably assist us with all of these tasks and remain hopeful AI can someday transition us to those endpoint scenarios.
Overall, we are not at a place where we can use AI to give prognostic indicators and replace our current therapeutic algorithms. Until we design and conduct clinical trials utilizing AI as the decision maker for actionable treatment decisions, (i.e., whether or not to perform SLNB, provide adjuvant therapy, or conduct surveillance radiologic imaging studies), we won’t be able to incorporate even the most accurate AI technology into our treatment algorithms.
Until we have that tangible data, we as dermatologists can continue to advocate for clinical evidence and randomized prospective clinical trials that will assist with the further development of AI technology applications to be used in the classification and management of melanoma.Â
REFERENCES
-
- Stiff, Katherine M., et al. “Artificial Intelligence and Melanoma: A Comprehensive Review of Clinical, Dermoscopic, and Histologic Applications.” Pigment Cell & Melanoma Research (2022).
- Cui, Xiaoyu, et al. “Assessing the effectiveness of artificial intelligence methods for melanoma: A retrospective review.” Journal of the American Academy of Dermatology 81.5 (2019): 1176-1180.
- Marchetti, Michael A., et al. “Performance of gene expression profile tests for prognosis in patients with localized cutaneous melanoma: a systematic review and meta-analysis.” JAMA dermatology 156.9 (2020): 953-962.
- Haenssle, Holger A., et al. “Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists.” Annals of oncology 29.8 (2018): 1836-1842.
- Brinker, Titus J., et al. “Comparing artificial intelligence algorithms to 157 German dermatologists: the melanoma classification benchmark.” European Journal of Cancer 111 (2019): 30-37.
- Phillips, Michael, et al. “Assessment of accuracy of an artificial intelligence algorithm to detect melanoma in images of skin lesions.” JAMA network open 2.10 (2019): e1913436-e1913436.
- Cook, Robert W., et al. “Analytic validity of DecisionDx-Melanoma, a gene expression profile test for determining metastatic risk in melanoma patients.” Diagnostic pathology 13.1 (2018): 1-8.
Image used with permission from the Journal of Drugs in Dermatology.Â
Did you enjoy this article? Find more on Artificial Intelligence (AI) in Dermatology here.