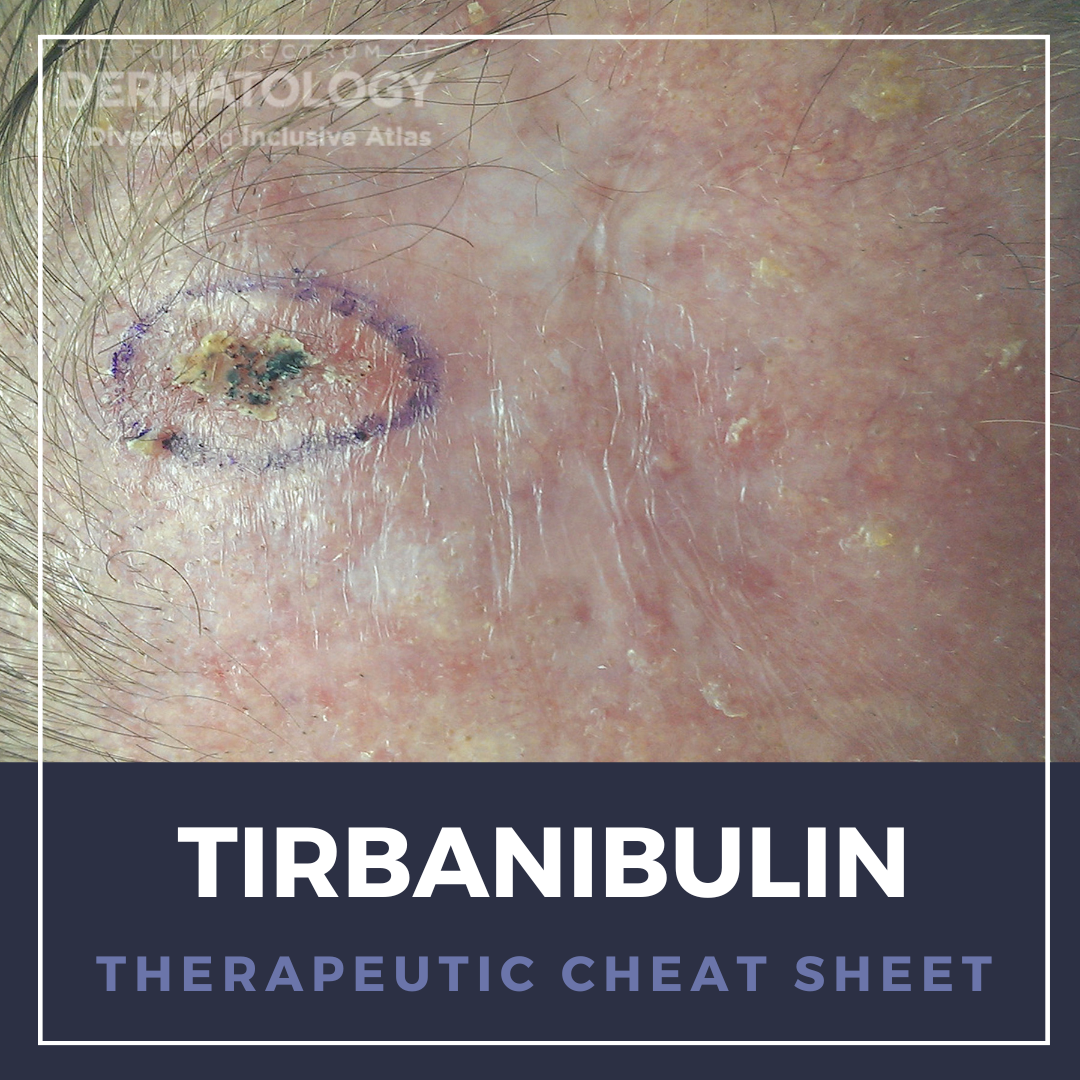
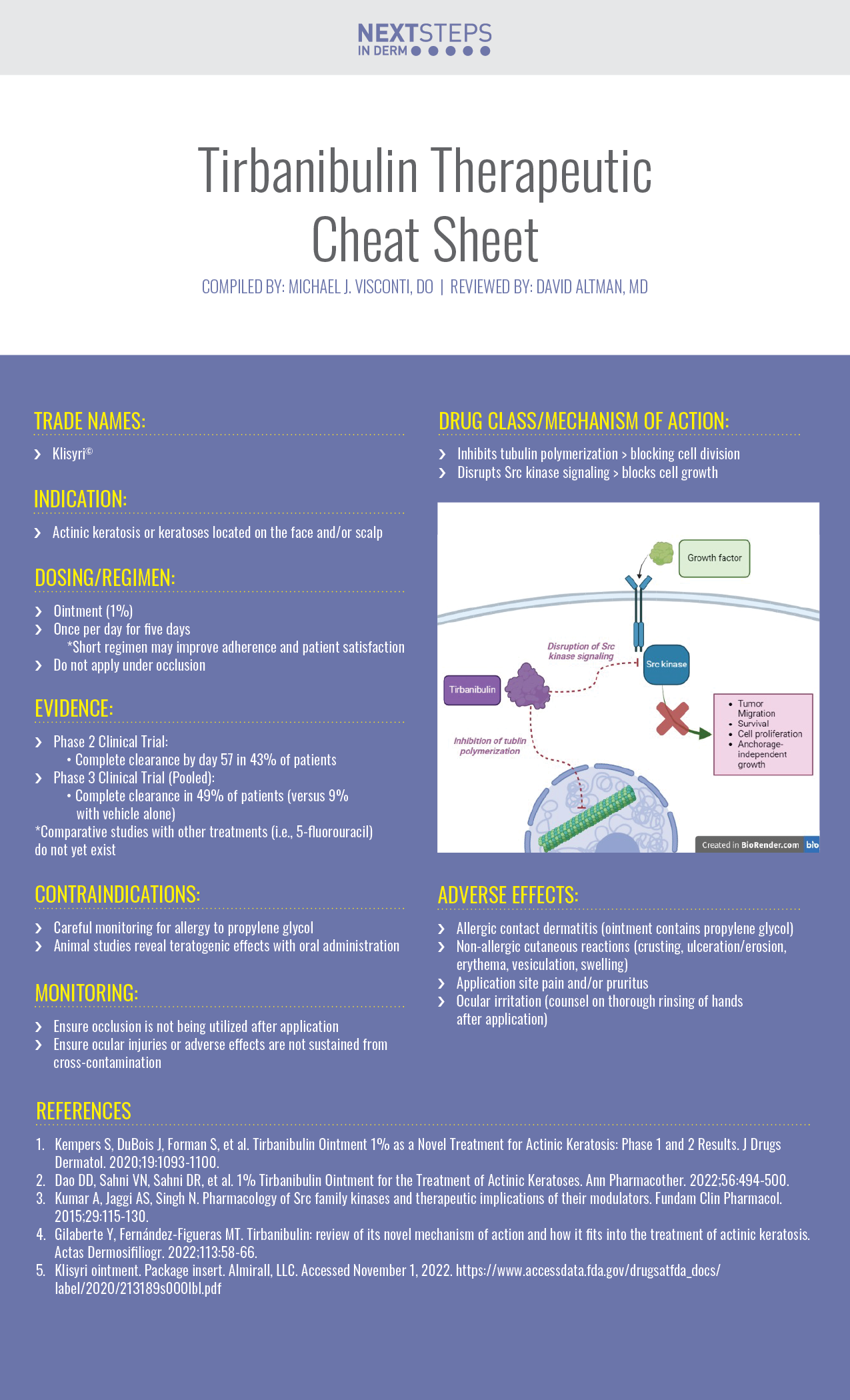
Current field-directed treatments of actinic keratosis (AK), a pre-malignant condition, are often limited by severe local reactions and/or complex treatment. Tirbanibulin, a novel potent anti-proliferative synthetic agent that inhibits tubulin polymerization and Src kinase signalling, offers a convenient, safe, and effective field treatment of actinic keratosis. We continue our surgical Therapeutic Cheat Sheet series with a closer look at Tirbanibulin, which is FDA-approved for the treatment of actinic keratosis (AK) of the face or scalp.
Further Reading
To learn more about Tirbanibulin, check out the following articles published in the Journal of Drugs in Dermatology (JDD):
Safety and Tolerability of Topical Agents for Actinic Keratosis: A Systematic Review of Phase 3 Clinical Trials
ABSTRACT
Background: Topical agents for actinic keratosis (AK), along with cryotherapy and phototherapy, are the most commonly used therapies for areas of skin with multiple AKs. Multiple options for the topical treatment of AK exist; newer therapies aim to balance efficacy with an acceptable safety and tolerability profile for the patient.
Objective: To describe the safety and tolerability of FDA-approved topical agents for the treatment of AK.
Methods: A systematic review of phase III clinical trials of topical agents for AK available on PubMed and clinicaltrials.gov was conducted on January 10th, 2021.
Results: 29 phase III clinical trials meeting the inclusion criteria were included in the qualitative synthesis. No serious adverse events or systemic adverse events were determined to be due to topical therapies for AK. The highest rates of treatment-related application-site adverse events and local skin reactions occurred with the various formulations of topical 5-FU and imiquimod; newer topical agents such as ingenol mebutate and tirbanibulin had more favorable tolerability profiles.
Conclusions: FDA-approved topical agents for the treatment of multiple AKs have minimal safety concerns. Tolerability profiles vary among the available options, and new agents such as tirbanibulin offer a favorable combination of safety, tolerability, and efficacy.
J Drugs Dermatol. 2021;20:10(Suppl):s4-11.
Tirbanibulin Ointment 1% as a Novel Treatment for Actinic Keratosis: Phase 1 and 2 Results
Background: Current field-directed treatments of actinic keratosis (AK), a pre-malignant condition, are often limited by severe local reactions and/or complex treatment. Tirbanibulin, a novel potent anti-proliferative synthetic agent that inhibits tubulin polymerization and Src kinase signalling, is being developed as a convenient, safe, and effective field treatment of actinic keratosis.
Hypothesis: A short course of tirbanibulin ointment 1% safely reduces AK lesions.
Methods: In the Phase 1 study, 4 treatment cohorts with forearm lesions received tirbanibulin ointment 1% over 25 or 100 cm2 once daily for 3 or 5 days and were evaluated through day 45. In the Phase 2 study, 2 treatment cohorts with face or scalp lesions received tirbanibulin ointment 1% once daily for 3 or 5 days over 25 cm2 and were evaluated through day 57. Lesion reductions, clearance rates, safety, and pharmacokinetics were assessed.
Results: Forearm AK lesions were reduced by day 45 in all Phase 1 cohorts (N=30). Complete AK clearance at day 57 for face/scalp AK lesions in Phase 2 cohorts (N=168) was demonstrated in 43% and 32% of participants of the 5-day and 3-day cohorts, respectively. Adverse reactions were mainly transient mild local erythema and flaking/scaling, pruritus, and pain. Tirbanibulin plasma concentrations were low or undetectable.
Conclusion: Tirbanibulin ointment 1% was well tolerated and active in AK reduction. Based on activity, the 5-day regimen was selected for Phase 3 development.
Clinicaltrials.gov: NCT02337205; NCT02838628
J Drugs Dermatol. 2020;19(11):1093-1100. doi:10.36849/JDD.2020.5576
Did you enjoy this Therapeutic Cheat Sheet? You can find more here.