Dr. Yasmine Kirkorian, Interim Chief of Dermatology at Children’s National Health System and Assistant Professor of Dermatology and Pediatrics at the George Washington School of Medicine & Health Sciences, summarizes recent controversies in the field of pediatric dermatology regarding the usage of systemic therapies in children.
This article will provide an overview of the following:
-
- What’s ON Label for Pediatric Skin Disease
- What’s in Clinical Trials for Pediatric Skin Disease
- What’s OFF Label but used in Pediatric Skin Disease
- Pearls about specific medications used in Pediatric Skin Disease
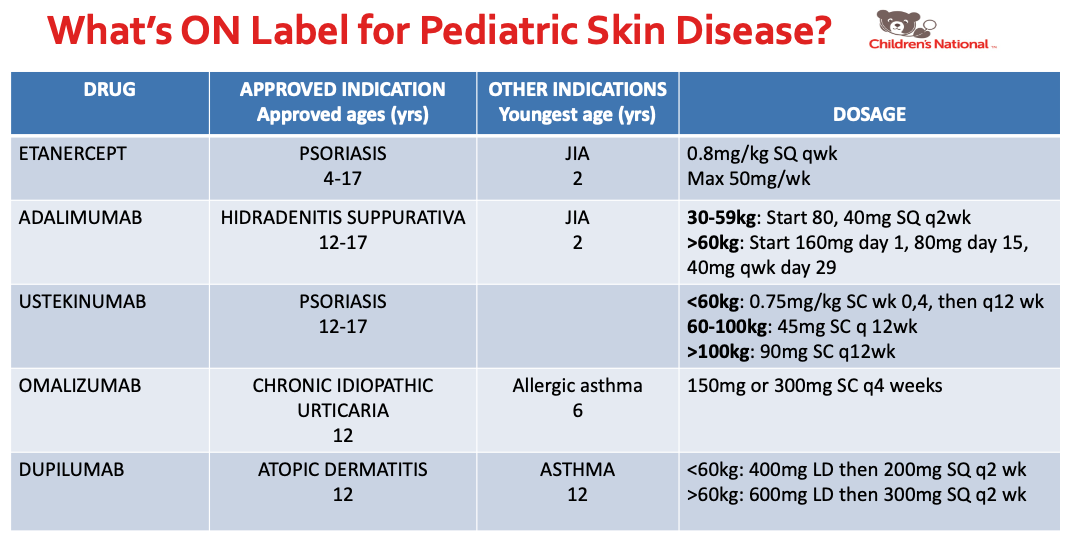
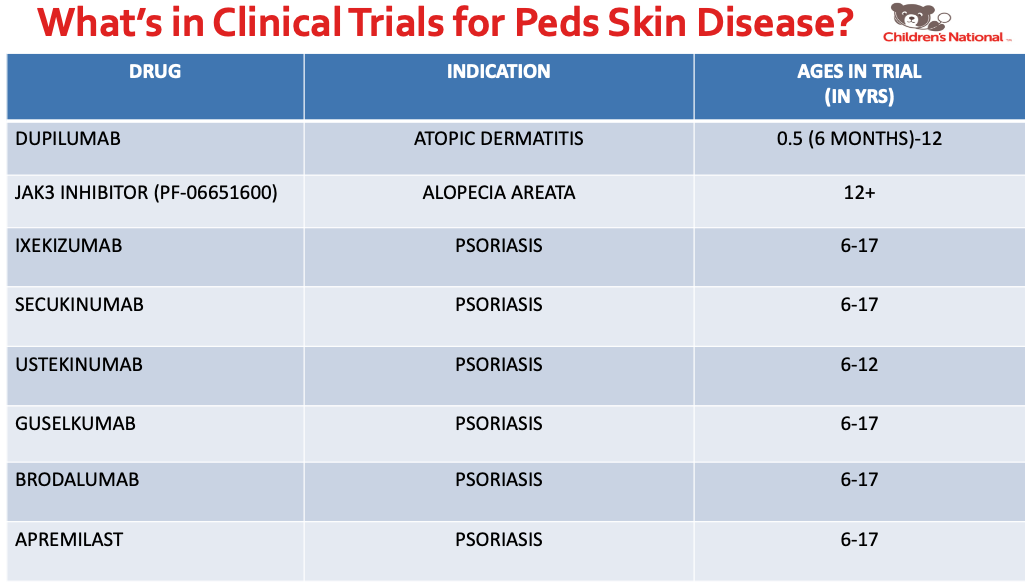
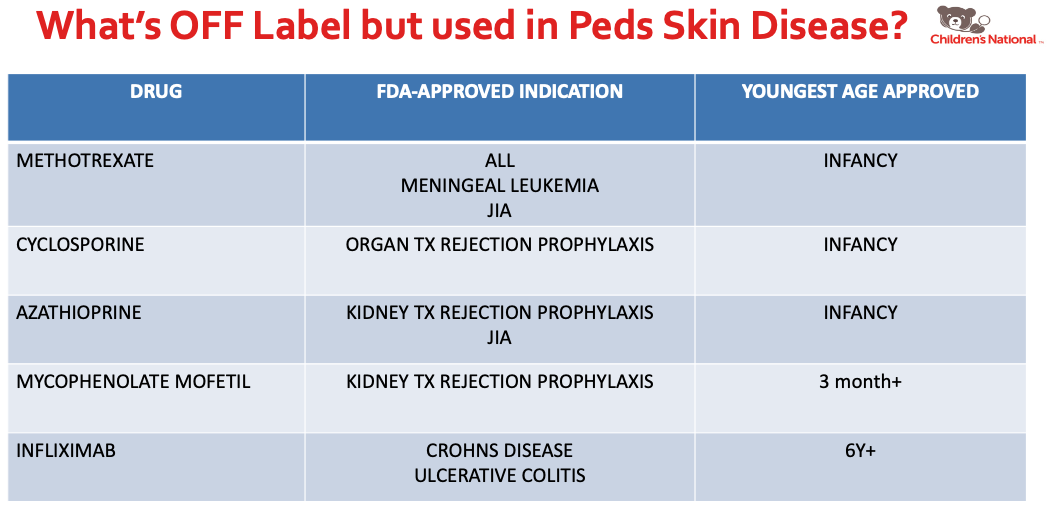
Dr. Kirkorian also discussed systemic medications used in pediatric atopic dermatitis. I learned about the following facts:
Methotrexate in atopic dermatitis
No loading dose is needed, dosing is given at 0.3-0.7mg/kg weekly, and a maximum dose of 25mg weekly was recommended. The medication is cheap, but shows a lack of efficacy in many. Children also experience less GI distress with folic acid 1 mg daily instead of weekly folic acid dosing.
Cyclosporine in atopic dermatitis
The dosing for this medication is 3-5mg/kg divided BID (Dr. Kirkorian recommend starting at 5mg/kg dosing). This medication is a fast acting medication for atopic dermatitis that acts as a bridge to long term treatment. It was also pointed out that school nurse can help do the blood pressure checks, which is a useful tip since cyclosporine requires long term blood pressure monitoring.
Dupilumab in atopic dermatitis
No lab draws, game changer for atopic dermatitis. Possible side effects include ocular surface disease, facial dermatitis, and alopecia.
Dr. Kirkorian also touched on drugs used on-label and off-label in pediatric psoriasis, and discussed vaccine administration in the age of biologics.
Overall, the main conclusions from Dr. Kirkorian’s lecture were as follows:
1. Pediatric patients with severe dermatological diseases have limited FDA approved options for systemic treatment, but this is changing.
2. In order to obtain insurance approval in the setting of no approved drug: demonstrate failure of other first-line agents and then ask for insurance approval based on clinical studies in the literature.
3. Insurance may require failure of an off-label systemic drug to approve an on-label systemic drug.
4. Live vaccines are generally contraindicated with most systemic medications in children. If possible, vaccinate/give boosters BEFORE starting biologics.
Source:
This information was presented by Dr. Yasmine Kirkorian at the 17th Annual ODAC Dermatology, Aesthetic and Surgical Conference held January 17–20, 2020 in Orlando, FL. The above highlights from her lecture were written and compiled by Dr. Olivia Lai, one of the 7 residents selected to participate in the Sun Young Dermatology Leader Mentorship Program (a program supported by an educational grant from Sun Pharmaceutical Industries, Inc.).
Image Credits:
Figures 1, 2, and 3 are courtesy of Yasmine Kirkorian, MD
Did you enjoy this post? Find more on Medical Dermatology here.

