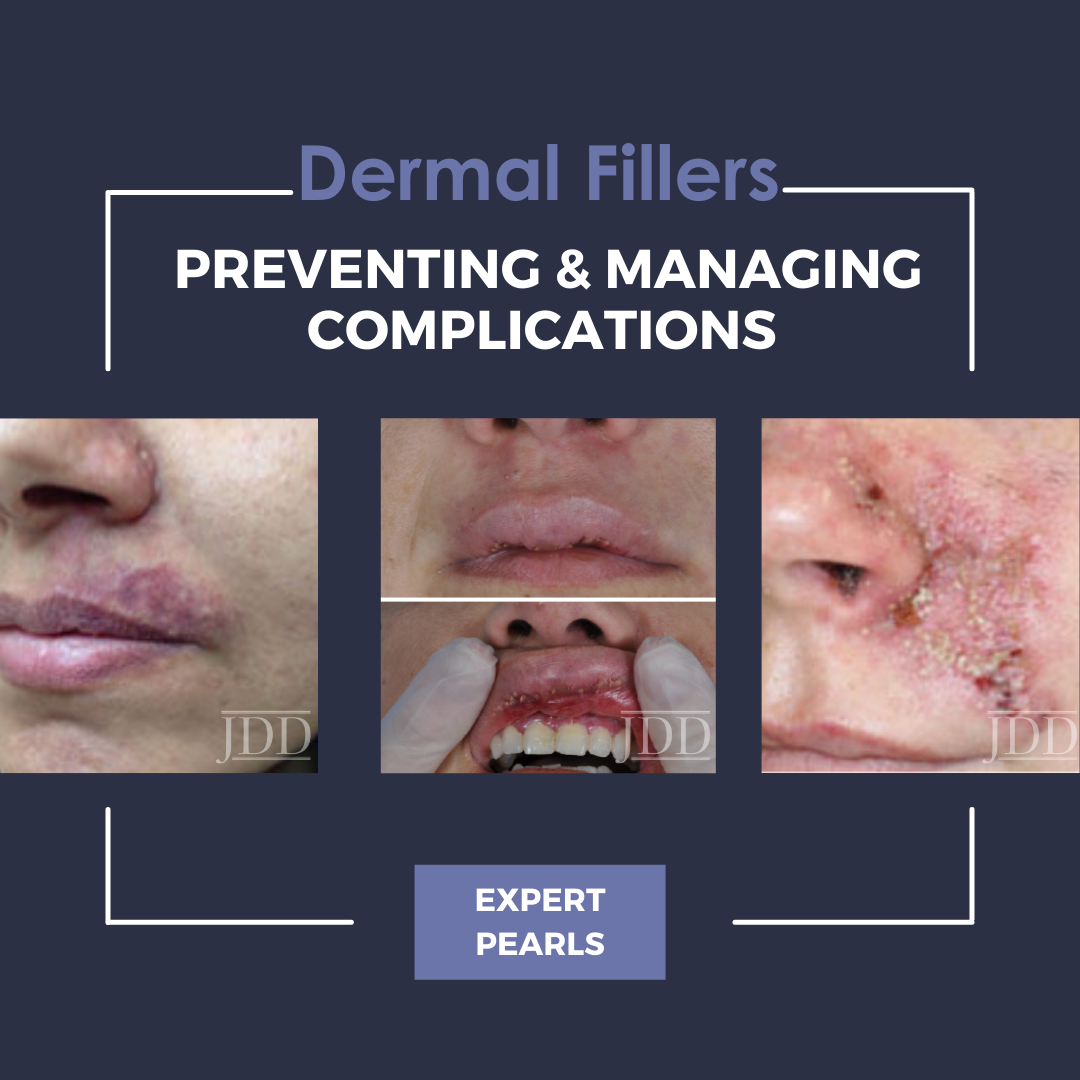
During the 2021 Skin of Color Update Virtual Conference, Dr. Hassan Galadari provided a highly anticipated lecture in which he discussed prevention and management of complications with dermal filler injections.
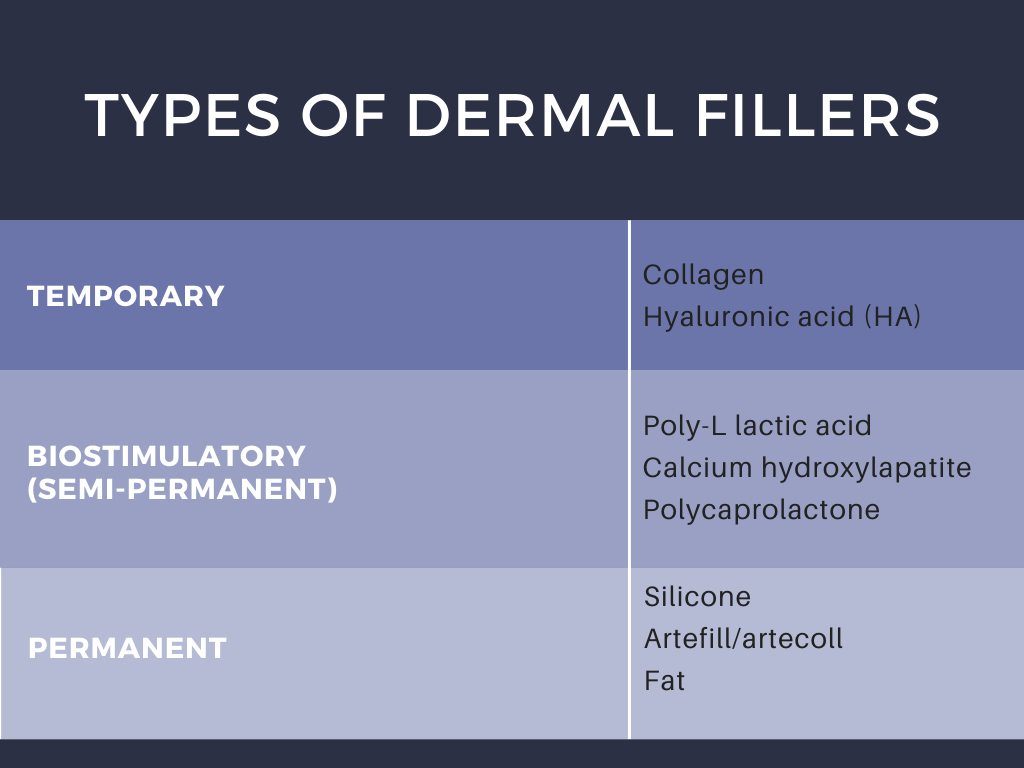
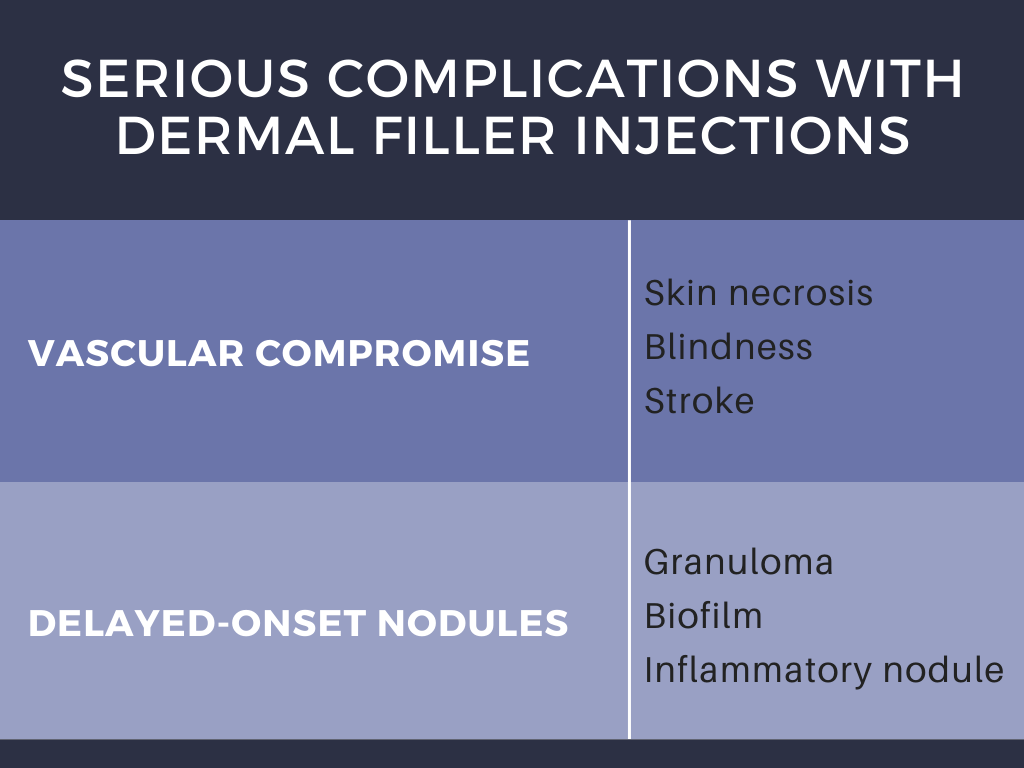
The different types of dermal filers, shown above in Table 1, have been associated with a variety of complications that have proven to occur only rarely (<1% patients) when injected by experienced practitioners.1 Dr. Galadari discussed results published by Povolotskiy et al, conveying that most adverse effects were relatively minor and transient.1 Authors investigated the safety of dermal filler injections by analyzing adverse events reported to the United States Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) from 2007 to 2017.1,2 The MAUDE passive surveillance system receives and compiles reports to monitor device performance and safety annually, providing valuable data on post-market surveillance of medical devices while raising awareness about device malfunction.1,2 Using this data, authors conveyed that serious complications including vascular compromise or late-occurring nodules may occur rarely (Table 2).1 Blindness is a rare complication, occurring in 0.94% of patients after injections in the nose, supraorbital area, and cheek.1 Infection and nodule formation are the most commonly occurring adverse events, occurring in 51.25% and 58.76% of patients, respectively.1 Despite the high prevalence of infection, sepsis only resulted in 0.68% of patients receiving dermal filler injections.1
This study also went on to classify complication by filler type, however this data is less robust given that the filler brands reported were the most popular in the United States alone, and it is likely that some of the reported fillers with comparatively fewer complications were either not utilized as frequently or had under-reporting of complications. Regardless of filler type, the general estimated rate of adverse events is about 1 in 3,600 cases.1
Adverse events can be alternatively sub-classified by timing of onset. Early-onset complications occur hours to days after injection, and include site reactions, hypersensitivity, infection, Tyndall effect, surface irregularities & nodules, vascular occlusion, and embolic phenomena. Symptoms of site reactions include edema, pain, erythema, ecchymosis, and itching. Types of infections that may occur include herpes simplex virus, abscess, cellulitis, or mycobacterial infection. The Tyndall effect manifests with blue light being reflected back to observers as a result of the material injected into the skin. Vascular occlusion and embolic phenomena can result in local tissue necrosis, blindness, and stroke as previously discussed. Delayed complications occur weeks to years later, and include late-occurring nodules, dyspigmentation, and scarring.
Complications may arise from a number of etiologies but are preventable and treatable. In addition to emphasizing the importance of choice of filler for each clinical situation, Dr. Galadari conveyed that poor injection technique (needle vs. cannula) and injection into or compression of a blood vessel may lead to increased complications.
VASCULAR COMPROMISE
Common triggering injection sites: Most common injection sites leading to blindness complication from dermal filler include: glabella (38.8%), nasal region (25.5%), nasolabial fold (13.3%), and forehead (12.2%).3 It is critical to understand the anatomy of these areas and to avoid dangerous injection zones.
Presentation: Immediate sharp pain, blanching. Reticulated erythema and purpura 3-6 hours later, with subsequent ulceration and scarring within days. Early recognition and treatment is important to avoid irreversible changes.
Treatment: Stop injecting, massage vigorously, use warm compresses 5 to 10 minutes.4,5 Repeat every 30 to 60 minutes. For HA fillers, use hyaluronidase 200-500 IU diluted in lidocaine or saline injected 2 to 3 cm apart and repeated every hour.4,5 Complete 3-4 cycles or stop when symptoms improve.4,5 Start aspirin 325mg BID for 2-3 days and consider sildenafil.3,4 Be careful with nitroglycerin, which can often lead to headaches.4,5
Prevention: Inject slowly under low pressure and keep the needle moving. Use small volumes and implement the micro-droplet technique. Another technique is to fractionate treatment across several different sessions. Using a cannula can also have benefits over injection with a needle, resulting in fewer occlusion events.4,5
DELAYED-ONSET NODULES
Inflammatory nodules are influenced by a number of dermal filler characteristics, including filler particle shape, surface change, hydrophilicity, and cross-linking. There are 3 potential causes for inflammatory nodules: 1) immune-mediated reactions, 2) foreign body responses (more common in non-biodegradable fillers), and 3) infectious processes (biofilms). Reported incidence of delayed-onset nodules is 0.5% and data suggests that less than half of occurrences have an identifiable immunological trigger.6 Median time from injection to reaction is 4 months.6 Median time to resolution is 6 weeks.6
Etiology: Low molecular weight (LMW) HA fragments have pro-inflammatory effects by inhibiting fibrocyte differentiation via the CD44 receptor.6,7 Interaction with CD44 also involved in inducing expression of pro-inflammatory cytokine IL-6 and chemokines IL-8, CXCL-1, CXCL-2, CXCL-6, and CCL-8 gene expression in normal human dermal fibroblasts. LMW HA can also activate macrophages, dendritic cells, and signal T-cells.7,8
Treatment: Oral and intralesional corticosteroids and hyaluronidase.
Prevention: Inject the material at the appropriate level, ice the target area prior to and after treatment, massage the treated area. Use the smallest gauge needle possible and avoid overcorrection.
BIOFILMS
Etiology: Biofilms consist of dense communities of bacteria that surround themselves with secreted polymers. These bacteria secrete an adhesive matrix allowing them to irreversibly adhere to a living structure or inert surface, where they give rise to a low-grade chronic infection resistant to antibiotics. Biofilm can cause a local infection, a systemic infection, or a granulomatous inflammatory response.
Presentation: Distinguishing inflammation due to bacterial biofilm from a low-grade hypersensitivity reaction is sometimes difficult. If a red, indurated area appears any time after treatment, a biofilm should be suspected. Cultures are usually negative. Fluorescence in situ hybridization can confirm an infectious etiology. Scintigraphy with radiolabeled autologous white blood cells is also an accurate method for diagnosing infection in patients with long-term dermal filler complications.
Treatment: Broad-spectrum antibiotics are helpful, and Dr. Galadari suggested a regimen consisting of ciprofloxacin 500mg BID and clarithromycin XL 500mg BID for 4-6 weeks. Hyaluronidase should be used if the patient was injected with HA. Intralesional steroids should not be used before antibiotics. If a long-term indurated area persists and if steroids have already been used, treatment with 5-FU injection (up to 50mg/mL, 0.5cc), alone or in combination with steroids can be helpful. Laser lysis or radiofrequency heat also helps decrease the bacterial count.
Prevention: Thorough cleansing of the face with chlorhexidine before injection, reducing number of punctures, avoiding injecting through oral or nasal mucosa, avoiding hydrophilic permanent materials, and avoiding injecting over previous filler or into traumatized tissue.
IN CONCLUSION
Dr. Galadari concluded his presentation with some important words of advice. It is critical to avoid products that do not have appropriate safety track records, to be aware of anticoagulants patients are using, and to use the appropriate filler for the correct area. After a complication occurs, it is advised to send the patient back to the practitioner who performed the initial procedure (if at all possible), in order to avoid misrepresenting your care as that which caused the complication. Second, treatment timing should be immediate when neurovascular compromise is considered. Lastly, if you are not comfortable treating a patient, it is better to refer them to someone else. Dr. Galadari’s lecture was a highly practical review of filler complications, and an invaluable resource to any practitioner performing filler injections.
References:
-
- Povolotskiy R, Oleck NC, Hatzis CM, Paskhover B. Adverse events associated with aesthetic dermal fillers: a 10-year retrospective study of FDA data. The American Journal of Cosmetic Surgery. 2018 Sep;35(3):143-51.
- MAUDE – Manufacturer and User Facility Device Experience (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm)
- Beleznay K, Carruthers JD, Humphrey S, Jones D. Avoiding and treating blindness from fillers: a review of the world literature. Dermatologic Surgery. 2015 Oct 1;41(10):1097-117.
- Cohen JL, Biesman BS, Dayan SH, DeLorenzi C, Lambros VS, Nestor MS, Sadick N, Sykes J. Treatment of hyaluronic acid filler–induced impending necrosis with hyaluronidase: consensus recommendations. Aesthetic Surgery Journal. 2015 Sep 1;35(7):844-9.
- Jones DH, Fitzgerald R, Cox SE, Butterwick K, Murad MH, Humphrey S, Carruthers J, Dayan SH, Donofrio L, Solish N, Yee GJ. Preventing and Treating Adverse Events of Injectable Fillers: Evidence-Based Recommendations From the American Society for Dermatologic Surgery Multidisciplinary Task Force. Dermatologic Surgery. 2021 Feb 1;47(2):214-26.
- Beleznay K, Carruthers JD, Carruthers A, Mummert ME, Humphrey S. Delayed-onset nodules secondary to a smooth cohesive 20 mg/mL hyaluronic acid filler: cause and management. Dermatologic Surgery. 2015 Aug 1;41(8):929-39.
- Maharjan AS, Pilling D, Gomer RH. High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PloS one. 2011 Oct 11;6(10):e26078.
- Cyphert JM, Trempus CS, Garantziotis S. Size matters: molecular weight specificity of hyaluronan effects in cell biology. International journal of cell biology. 2015 Oct;2015.
Images used with permission from the Journal of Drugs in Dermatology.
This information was presented by Dr. Hassan Galadari at the 2021 Skin of Color Update virtual conference held on September 10-12, 2021. The above highlights from his lecture were written and compiled by Dr. Azam Qureshi.
Did you enjoy this article? Find more on Aesthetic Dermatology here.