Baricitinib Therapeutic Cheat Sheet
 Baricitinib (Olumiant®) is a once daily oral medication recently FDA approved for severe alopecia areata, which is defined as having a Severity of Alopecia Tool (SALT) score of 50 or higher.1 JAK inhibitors are a relatively new class of drug demonstrating efficacy and safety in a range of inflammatory skin disorders. Emerging studies have highlighted baricitinib’s effectiveness for conditions l …
Baricitinib (Olumiant®) is a once daily oral medication recently FDA approved for severe alopecia areata, which is defined as having a Severity of Alopecia Tool (SALT) score of 50 or higher.1 JAK inhibitors are a relatively new class of drug demonstrating efficacy and safety in a range of inflammatory skin disorders. Emerging studies have highlighted baricitinib’s effectiveness for conditions l …
 Baricitinib (Olumiant®) is a once daily oral medication recently FDA approved for severe alopecia areata, which is defined as having a Severity of Alopecia Tool (SALT) score of 50 or higher.1 JAK inhibitors are a relatively new class of drug demonstrating efficacy and safety in a range of inflammatory skin disorders. Emerging studies have highlighted baricitinib’s effectiveness for conditions l …
Baricitinib (Olumiant®) is a once daily oral medication recently FDA approved for severe alopecia areata, which is defined as having a Severity of Alopecia Tool (SALT) score of 50 or higher.1 JAK inhibitors are a relatively new class of drug demonstrating efficacy and safety in a range of inflammatory skin disorders. Emerging studies have highlighted baricitinib’s effectiveness for conditions l … 

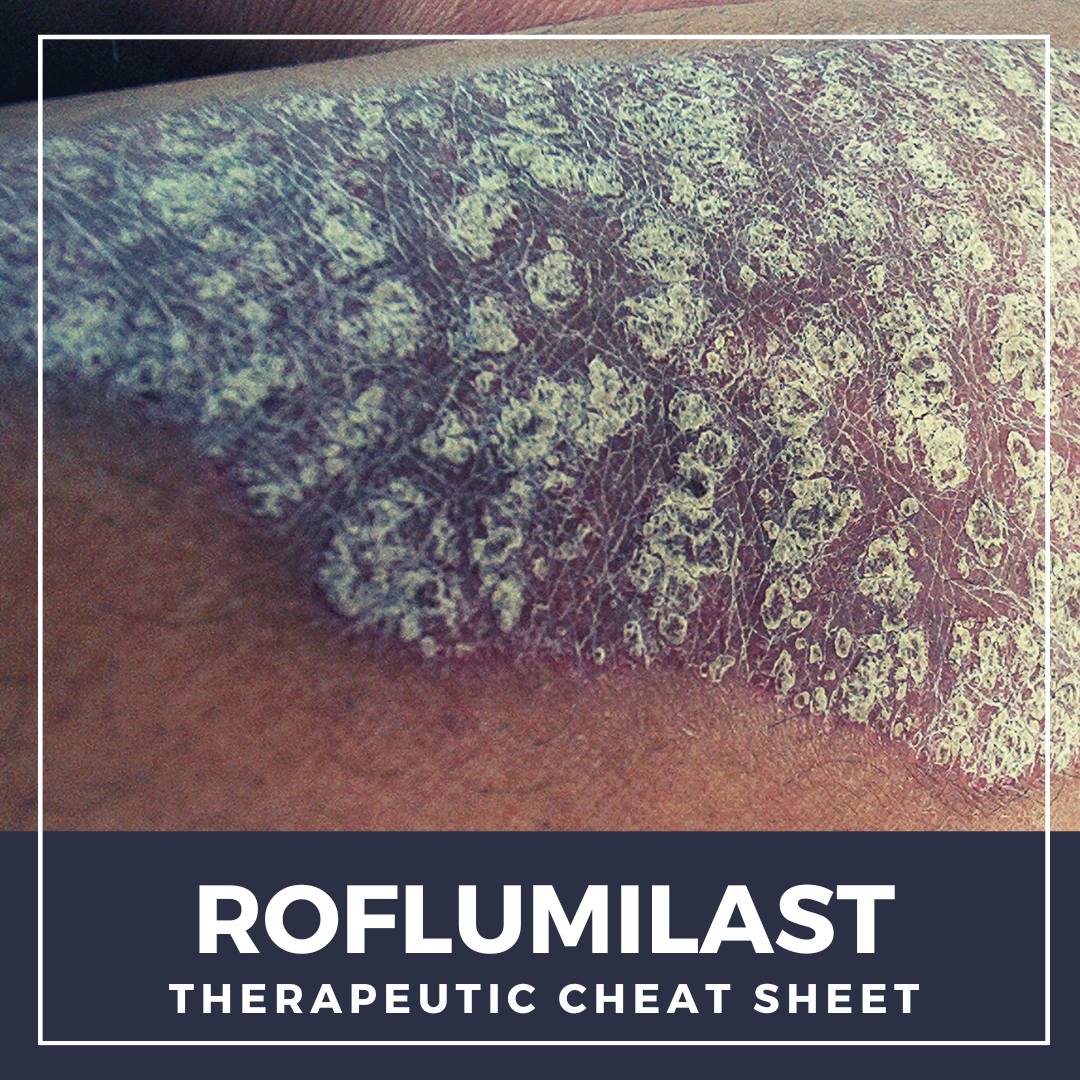 Roflumilast is a non-steroidal topical cream that was FDA approved for the treatment of plaque psoriasis in 2022.1 Roflumilast selectively inhibits phosphodiesterase-4 (PDE-4), which is an enzyme that mediates inflammatory responses and has been shown to play a role in several dermatologic diseases.2 Prior to topical roflumilast, oral PDE-4 inhibitors have been investigated across a wide variety o …
Roflumilast is a non-steroidal topical cream that was FDA approved for the treatment of plaque psoriasis in 2022.1 Roflumilast selectively inhibits phosphodiesterase-4 (PDE-4), which is an enzyme that mediates inflammatory responses and has been shown to play a role in several dermatologic diseases.2 Prior to topical roflumilast, oral PDE-4 inhibitors have been investigated across a wide variety o … 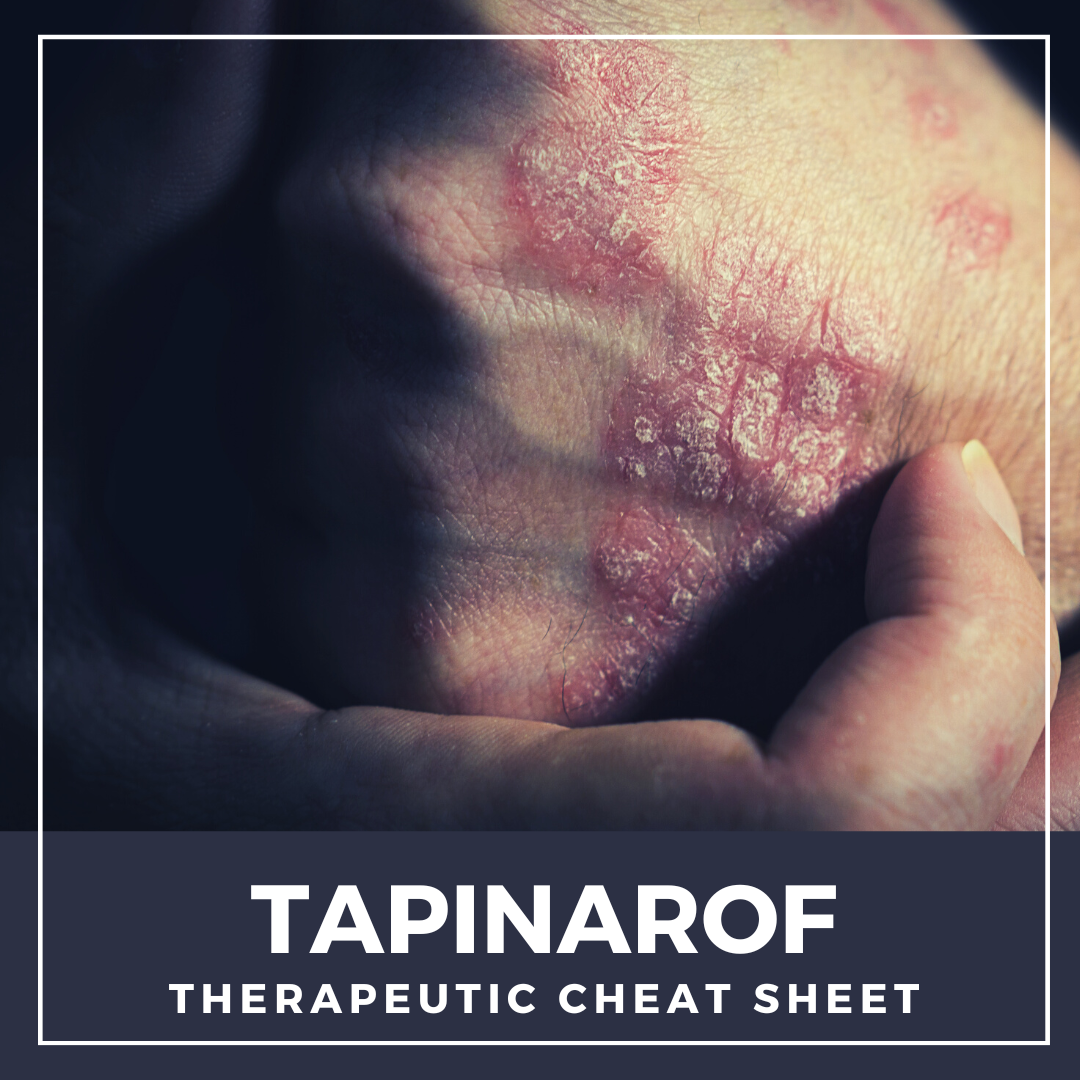 Tapinarof cream is a non-steroidal topical medication that was recently FDA approved in 2022 for the treatment of mild, moderate, or severe plaque psoriasis in adult patients. As biologic medications have continued to grow and become exceedingly present in the treatment of moderate to severe psoriasis, there has been very minimal, if any innovation in the space of topical treatments. Additionally, …
Tapinarof cream is a non-steroidal topical medication that was recently FDA approved in 2022 for the treatment of mild, moderate, or severe plaque psoriasis in adult patients. As biologic medications have continued to grow and become exceedingly present in the treatment of moderate to severe psoriasis, there has been very minimal, if any innovation in the space of topical treatments. Additionally, …  Azelaic acid is a topical therapeutic agent which is FDA approved to treat papules and pustules of mild to moderate rosacea and mild to moderate acne vulgaris. It was first approved by the FDA in 1995 and since its approval, has been used for many off-label conditions including disorders of hyperpigmentation. Its utility in various conditions can be attributed to its anti-microbial, anti-inflamm …
Azelaic acid is a topical therapeutic agent which is FDA approved to treat papules and pustules of mild to moderate rosacea and mild to moderate acne vulgaris. It was first approved by the FDA in 1995 and since its approval, has been used for many off-label conditions including disorders of hyperpigmentation. Its utility in various conditions can be attributed to its anti-microbial, anti-inflamm … 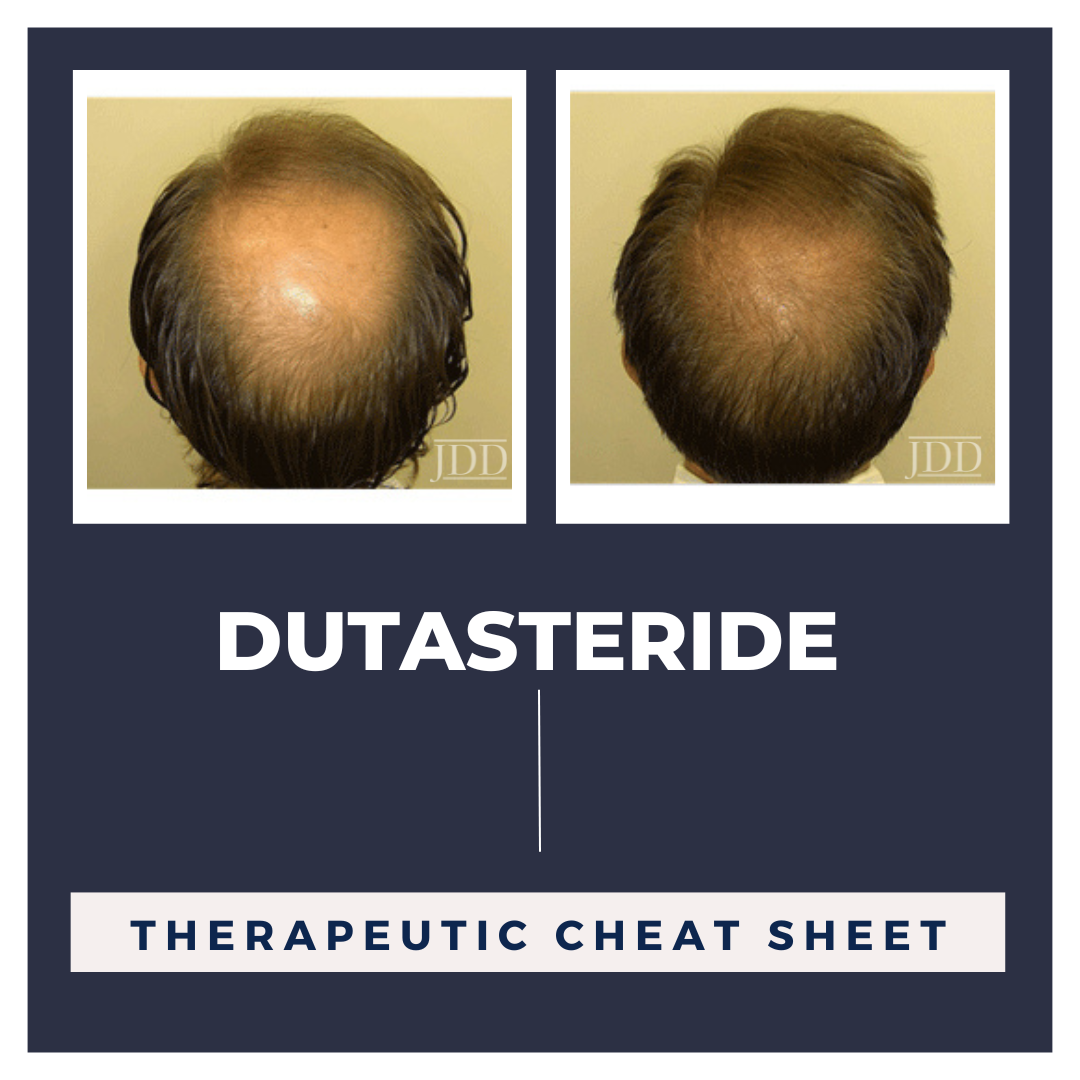 Androgenic alopecia (AGA) is one of the most common forms of hair loss and represents a frequently faced disabling concern in dermatology visits. Targeting the 5α-dihydrotestosterone (DHT) pathway has been shown to be an efficacious mechanism of action, with finasteride being the only systemic FDA-approved drug to treat male AGA. Recently, its sister drug, dutasteride, has been increasingly utili …
Androgenic alopecia (AGA) is one of the most common forms of hair loss and represents a frequently faced disabling concern in dermatology visits. Targeting the 5α-dihydrotestosterone (DHT) pathway has been shown to be an efficacious mechanism of action, with finasteride being the only systemic FDA-approved drug to treat male AGA. Recently, its sister drug, dutasteride, has been increasingly utili …