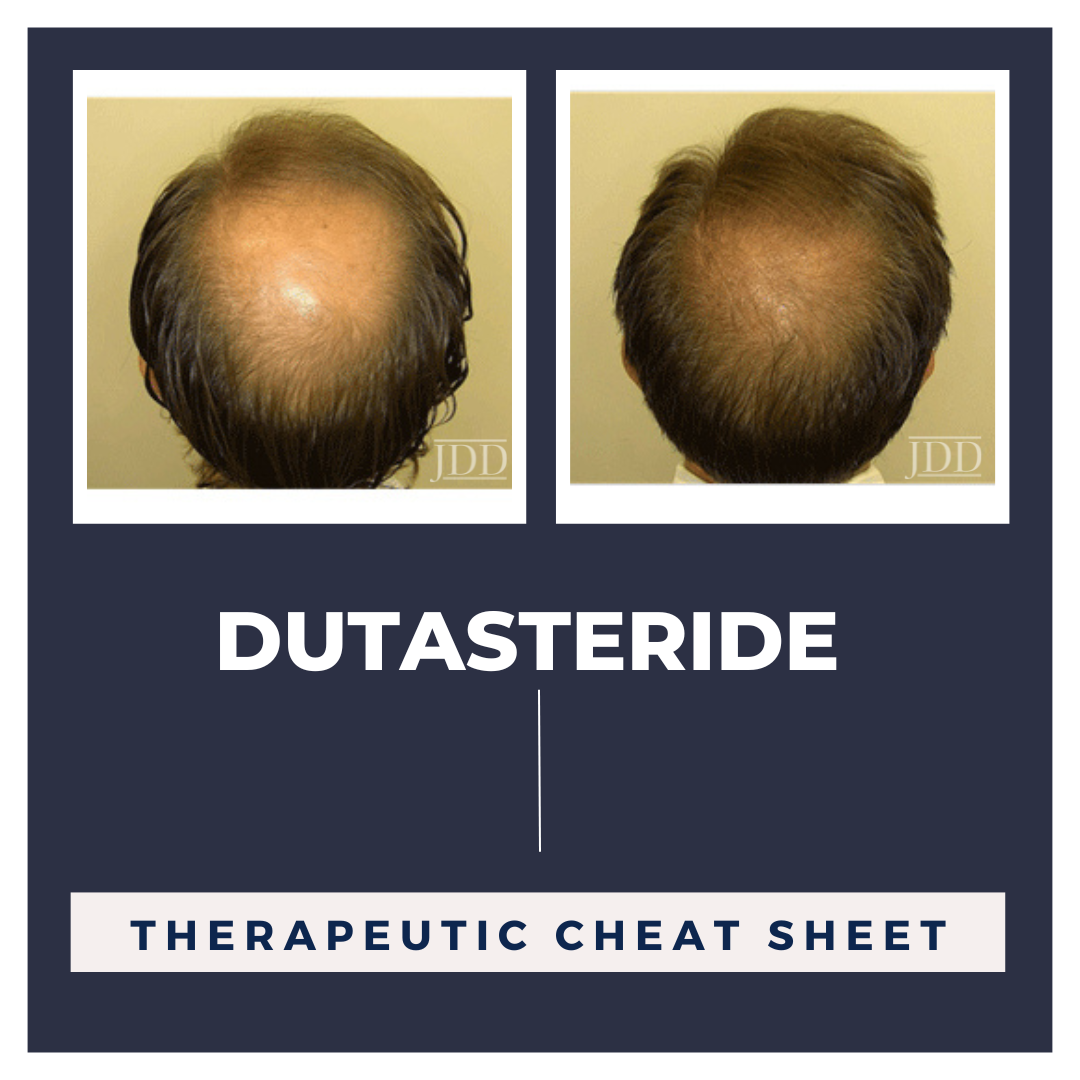
Androgenic alopecia (AGA) is one of the most common forms of hair loss and represents a frequently faced disabling concern in dermatology visits. Targeting the 5α-dihydrotestosterone (DHT) pathway has been shown to be an efficacious mechanism of action, with finasteride being the only systemic FDA-approved drug to treat male AGA. Recently, its sister drug, dutasteride, has been increasingly utilized given it’s increased potency in comparison to finasteride in inhibiting types 1 & 2 5α-reductase, while having a similar, well-tolerated side effect profile.1,2 We continue our series, Therapeutic Cheat Sheet, with a closer look at dutasteride, which is used extensively off-label for AGA along with frontal fibrosing alopecia and hidradenitis suppurativa.
Dutasteride Therapeutic Cheat Sheet
Compiled by: Azam Qureshi, MD | Reviewed by: Adam Friedman, MD
DUTASTERIDE TRADE NAME
-
- Avodart (oral)3
MECHANISM OF ACTION
-
- Synthetic 4-azasteroid compound, selectively inhibits type 1 and type 2 5α-reductase, thereby reducing conversion from testosterone to 5α-dihydrotestosterone (DHT)3
FDA APPROVED FOR
-
- Benign prostatic hyperplasia
OFF-LABEL USES OF DUTASTERIDE
-
- Androgenic alopecia (AGA)
- Frontal fibrosing alopecia (FFA)
- Hidradenitis suppurativa
- Possible utility in treating hirsutism, acne vulgaris in females*
*current literature supports possible benefit from sister drug finasteride for these conditions, but paucity of studies have investigated dutasteride directly4,5
DUTASTERIDE DOSING
-
- AGA, FFA
- Oral: 0.02 – 2.5mg daily, most commonly utilized dose is 0.5mg dutasteride daily in order to maximize efficacy while limiting side effects6,7
- Mesotherapy: 0.01%, 0.05%, 0.005% dutasteride8
- Topical: 0.01% dutasteride solution9
- Hidradenitis suppurativa
- 5mg dutasteride daily10
- AGA, FFA
DUTASTERIDE SIDE EFFECTS3
-
- Headaches
- Depression
- Nausea
- Hot flashes
- Decreased libido
- Reduced intensity of orgasm
- Impotence
- Abnormal ejaculation
- Gynecomastia
- Decrease in serum PSA levels
- Decreases risk of prostate cancer overall, may increase risk of high grade prostate cancer, however this is controversial given conflicting evidence in the literature
- Possible association with breast cancer and ovarian cancer, however lack of sufficient studies available
DUTASTERIDE WARNINGS
-
- Avoid use in patients with history of estrogen-mediated malignancies such as breast or ovarian cancer, however association is not well-supported
- Given reduction in serum PSA levels associated with dutasteride use, any PSA values obtained on therapy until 6 months after termination of therapy should be doubled for comparison with normal ranges in untreated men3
DUTASTERIDE CONTRAINDICATIONS
-
- Pregnancy
- Pediatric patients
- Patients with known hypersensitivity to dutasteride or other 5α-reductase inhibitors
PREGNANCY & BREASTFEEDING3
-
- Contraindicated in pregnancy, preclinical data suggests exposure to pregnant mother may inhibit development of male genital organs of a male fetus
- It is unknown if dutasteride is excreted in human breast milk
MONITORING
-
- No recommended blood monitoring guidelines
- Evaluation for prostate cancer should be performed on patients with BPH prior to initiating therapy and periodically afterwards
CLICK ON THE IMAGE BELOW TO ENLARGE/DOWNLOAD
Dutasteride has been increasingly used by dermatologists in recent years given its off-label utility in treating androgen-mediated skin disease including androgenic alopecia (AGA), one of the most common conditions encountered in dermatologist offices daily. In addition to AGA, dutasteride has been shown to be beneficial for frontal fibrosing alopecia and hidradenitis suppurativa. Although this medication is most commonly administered orally, topical therapy or mesotherapy using dutasteride may also be beneficial for these conditions. Practitioners should continue to take note of newly published reports concerning the expanding number of off-label uses of this medication. If you would like to learn more about dutasteride, check out the following 2 articles published in the Journal of Drugs in Dermatology:
Saceda-Corralo D, Moustafa F, Moreno-Arrones Ó, Jaén-Olasolo P, Vañó-Galván S, Camacho F.
Abstract:
Background: 5-alpha inhibitors are an effective treatment for androgenetic alopecia. Mesotherapy with dutasteride has been proposed as an effective method to improve hair loss and reducing systemic absorption.
Objective: The main objective was to describe the safety profile of mesotherapy with dutasteride in real clinical practice in a large cohort of patients with androgenetic alopecia. A secondary aim was to describe the effectiveness of this treatment.
Methods and materials: A multicentric retrospective study was designed. Patients treated with at least 6 months of follow-up were included in the study. Side effects and response to the treatment were analyzed.
Results: A total of 541 patients were included. The commonest approach during the first year was to perform the treatment every 3 months. Response to the mesotherapy in monotherapy could be assessed in 86 patients (15.9%) after one year. Most of them presented clinical improvement, being a marked improvement in 33 patients (38.4%). Pain was the most frequent side effect of the treatment (246 patients, 45.5%). No serious or sexual adverse events were detected.
Conclusion: Mesotherapy with dutasteride was effective in male and female hair loss in real clinical practice. Side effects related to the treatment were mild and self-limited. This therapy may be an effective option for select patients wishing to avoid oral treatment. J Drugs Dermatol. 2022;21(7):742-747. doi:10.36849/JDD.6610.
Side Effects Related to 5 α-Reductase Inhibitor Treatment of Hair Loss in Women: A Review
Seale LR, Eglini AN, McMichael AJ
Abstract:
5 α-reductase inhibitors such as finasteride and dutasteride have been studied for the treatment of hair loss in men, with finasteride being the only Food and Drug Administration-approved treatment. Increasingly, in recent years, off-label use of these drugs has been employed in the treatment of female pattern hair loss (FPHL) and frontal fibrosing alopecia (FFA) in women. Side effects with 5 α-reductase inhibitors can include changes in sexual function, and recent publications have characterized an increasing prevalence of these in men. A review of 20 peer-reviewed articles found that very few side effects, or adverse events, related to sexual function have been reported in studies in which dutasteride or finasteride has been used to treat hair loss in women. Future publications should investigate not only the efficacy of these drugs in treating FPHL and FFA, but the side effect profile in patients as well.
References
-
- Shanshanwal S, Dhurat R. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: A randomized controlled open-label, evaluator-blinded study. Indian journal of dermatology, venereology and leprology. 2017;83(1).
- Zhou Z, Song S, Gao Z, Wu J, Ma J, Cui Y. The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and meta-analysis. Clinical interventions in aging. 2019;14:399.
- GlaxoSmithKline: AVODART® (dutasteride) Soft Gelatin Capsules. Package Insert. US Prescribing Information and Patient Information. Available at https://gskpro.com/content/dam/global/hcpportal/en_NA/PI/Avodart-Soft-Caps-GCT002.pdf
- Rokni GR, Mohammadnezhad F, Saeedi M, Shadi S, Sharma A, Sandhu S, Gupta A, Goldust M. Efficacy, tolerability, and safety of montelukast versus finasteride for the treatment of moderate acne in women: A prospective, randomized, single‐blinded, active‐controlled trial. Journal of Cosmetic Dermatology. 2021 Nov;20(11):3580-5.
- Townsend KA, Marlowe KF. Relative safety and efficacy of finasteride for treatment of hirsutism. Annals of Pharmacotherapy. 2004 Jun;38(6):1070-3.
- Herz-Ruelas ME, Álvarez-Villalobos NA, Millán-Alanís JM, de León-Gutiérrez H, Ocampo-Garza SS, Gómez-Flores M, Grimalt R. Efficacy of Intralesional and Oral Dutasteride in the Treatment of Androgenetic Alopecia: A Systematic Review. Skin Appendage Disord. 2020 Nov;6(6):338-345. doi: 10.1159/000510697. Epub 2020 Oct 9. PMID: 33313048; PMCID: PMC7706484.
- Pindado-Ortega C, Saceda-Corralo D, Moreno-Arrones ÓM, Rodrigues-Barata AR, Hermosa-Gelbard A, Jaén-Olasolo P, Vañó-Galván S. Effectiveness of dutasteride in a large series of patients with frontal fibrosing alopecia in real clinical practice. Journal of the American Academy of Dermatology. 2021 May 1;84(5):1285-94.
- Saceda-Corralo D, Moustafa F, Moreno-Arrones Ó, Jaén-Olasolo P, Vañó-Galván S, Camacho F. Mesotherapy with dutasteride for androgenetic alopecia: a retrospective study in real clinical practice. Journal of drugs in dermatology: JDD. 2022 Jul 1;21(7):742-7.
- Sánchez-Meza E, Ocampo-Candiani J, Gómez-Flores M, Herz-Ruelas ME, Ocampo-Garza J, Orizaga-Y-Quiroga TL, Martínez-Moreno A, Ocampo-Garza SS. Microneedling plus topical dutasteride solution for androgenetic alopecia: a randomized placebo-controlled study. Journal of the European Academy of Dermatology and Venereology: JEADV.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatology online journal. 2013 Apr 1;19(4).
- Bienenfeld A, Azarchi S, Sicco KL, Marchbein S, Shapiro J, Nagler AR. Androgens in women: Androgen-mediated skin disease and patient evaluation. Journal of the American Academy of Dermatology. 2019 Jun 1;80(6):1497-506.
- Saceda-Corralo D, Moustafa F, Moreno-Arrones Ó, Jaén-Olasolo P, Vañó-Galván S, Camacho F. Mesotherapy with dutasteride for androgenetic alopecia: a retrospective study in real clinical practice. Journal of drugs in dermatology: JDD. 2022 Jul 1;21(7):742-7.
- Seale LR, Eglini AN, McMichael AJ. Side Effects Related to 5 α-Reductase Inhibitor Treatment of Hair Loss in Women: A Review. Journal of drugs in dermatology: JDD. 2016 Apr 1;15(4):414-9.
Did you enjoy this Therapeutic Cheat Sheet? You can find more here.