Dr. Lester is the Director of the Skin of Color Program at the Department of Dermatology University of California San Francisco. Wow! This means we got a “two for one special” from her lecture, not only learning about the truths and myths associated with the use of isotretinoin in acne but also recognizing that those who are underrepresented minorities or socioeconomically disadvantaged may not get isotretinoin when they need it!
Before we get started, let me share with you some of my “aha” moments from the renowned Dr. Lester’s lecture:
 You don’t have to check labs for isotretinoin every month! Get LFTs, CBC, Total Cholesterol, and Triglycerides at baseline, then 2 months into treatment. If they are normal both times, you may not need to check again!
You don’t have to check labs for isotretinoin every month! Get LFTs, CBC, Total Cholesterol, and Triglycerides at baseline, then 2 months into treatment. If they are normal both times, you may not need to check again!
 Suicide risk is elevated at baseline in acne patients and may be increased up to six months after discontinuing isotretinoin. Fortunately, some studies have shown that isotretinoin may actually decrease depression and suicidal ideation/attempts. We should carefully monitor our acne patients given their increased risk of depression and anxiety.
Suicide risk is elevated at baseline in acne patients and may be increased up to six months after discontinuing isotretinoin. Fortunately, some studies have shown that isotretinoin may actually decrease depression and suicidal ideation/attempts. We should carefully monitor our acne patients given their increased risk of depression and anxiety.
 You may want to avoid full-face dermabrasion and ablative laser therapy for 6+ months after discontinuing isotretinoin. There are different guidelines, so any elective procedure (even waxing!) should be discussed with the patient on a case-by-case basis.
You may want to avoid full-face dermabrasion and ablative laser therapy for 6+ months after discontinuing isotretinoin. There are different guidelines, so any elective procedure (even waxing!) should be discussed with the patient on a case-by-case basis.
So let’s read more about isotretinoin and the myths and misconceptions surrounding this medication.
Retinoids are synthetic and natural compounds that have biologic activity similar to Vitamin A1 . Isotretinoin, or 13-cis retinoic acid, is a first-generation retinoid that was approved by the US FDA in 1982 to treat severe nodulocystic acne. It works in the skin to inhibit sebocyte proliferation, differentiation, and lipid synthesis, reduce inflammation, decrease P. Acnes, and reduce hyperkeratinization1. Isotretinoin has been well described for its efficacy in treating severe and/or recalcitrant nodulocystic acne. Likewise, it has also earned a reputation for its side effects and robust iPLEDGE requirements given its teratogenicity.
So let’s play Myth Busters– Dermatology Style!
#1 | FACT OR FICTION?
While on Isotretinoin, the following should be checked monthly: CBC, AST, ALT, Total Bilirubin, Alkaline Phosphatase, Total Cholesterol, and Triglycerides.
FICTION
There is no definitive consensus on lab monitoring while on isotretinoin. What should we be scared about? High Triglyceride levels can lead to acute pancreatitis, which is fatal in about 5% of cases2. Typically triglyceride levels >1000mg/dL are associated with a significant risk of pancreatitis (5% risk), with the risk progressively rising in levels>500mg/dL3. Serious hematologic side effects of isotretinoin include anemia, agranulocytosis, neutropenia, and thrombocytopenia. We also worry about inflammation of the liver as well as rhabdomyolysis. These are all great reasons to check complete blood count (CBC) with liver function and lipid panels.
So now that the scary stuff is out of the way, we can talk about the more common, mild side effects: alopecia, cheilosis, dermatitis, dry skin, photosensitivity, pruritus, headache, dry eyes/nose bloods4. Decreased HDL level can be seen in up to 15% of patients, hypertriglyceridemia in 25-50% of cases, and elevated serum cholesterol in 7-30% . Elevated LFTs occur in up to 15%, musculoskeletal pain in 16% and in pediatric patients we can see up to 22% with arthralgia and 29% with backache1.
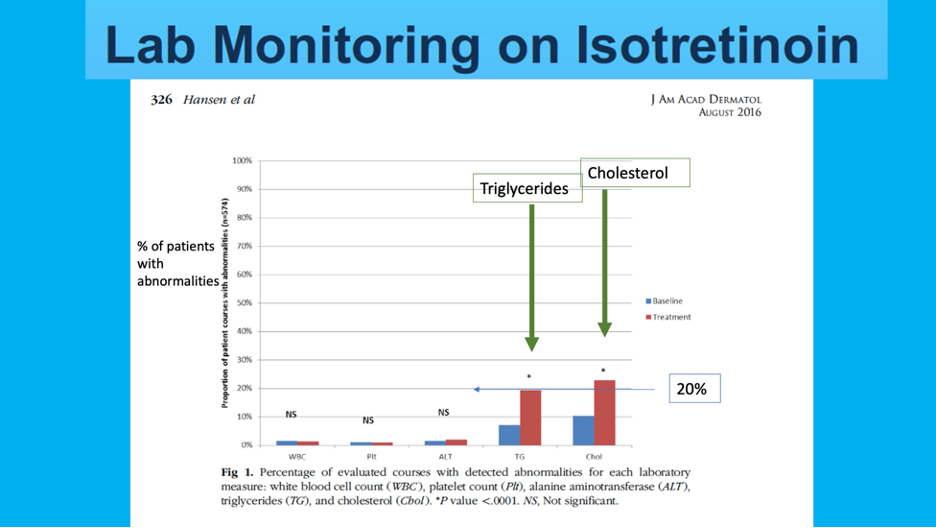
Check out Dr. Lester’s (modified) slide below. This slide shows a key figure from a 2016 JAAD article highlighting the incidence of laboratory abnormalities within a retrospective cohort of 515 patients undergoing 574 courses of isotretinoin5. As we can see, only triglycerides (TG) and cholesterol (Chol) showed statistically significant increases in patients while on isotretinoin therapy (19.3% and 22.8% increases from baseline, respectively). Only 0.9-1.4% of cases showed hematologic abnormalities and 1.9% LFT abnormalities. The mean time to lab abnormality detection was 50-61 days.
So what should we do in clinical practice?
Dr. Lester recommends that we should get baseline labs (CBC, Total cholesterol, Triglycerides, AST, ALT, and total bilirubin) and then recheck triglycerides and ALT 1 month after the max dose is achieved (or about two months into therapy). If these labs are normal, Dr. Lester does not check again.
#2 | FACT OR FICTION?
There is an increased suicide risk associated with Isotretinoin use.
FACT… AND FICTION…
Wuh? Okay, let’s break it down.
Patients on isotretinoin are more likely to be vulnerable to mental health concerns. Isotretinoin can be associated with aggressive behavior, depression, suicide ideation/attempt, psychotic disorder, and violent behavior. Depression, anxiety, and emotional lability are more commonly reported than suicidal ideation/attempt. Let’s examine the facts.
A 2010 study published in the British Medical Journal observed 5,756 patients with severe acne before, during, and after receiving isotretinoin6. Of those patients, 128 were admitted to the hospital for a suicide attempt. Even 1 year prior to treatment, the standardized incidence ratio (observed number divided by expected number based on age, gender, calendar year) was elevated in these patients with severe acne (although this number was not statistically significant). During the isotretinoin treatment course and up to 6 months after discontinuation, the standardized incidence ratio remained elevated 1.78 (95% CI 1.04-2.85) for all attempts, and 1.93 (95% CI 1.08-3.18) for first attempts only, both of which were statistically significant. Patients with a prior history of suicide attempt made new attempts to a lesser extent than those who made their initial attempts during or after isotretinoin therapy. Based on these findings, authors suggested that a history of suicide attempt should not preclude a patient from starting isotretinoin.
A 2019 JAMA Dermatology Article reviewed the FDA Adverse Event Reporting system for all reports of psychiatric adverse events related to isotretinoin from 1997-20177. They found 17,829 reported events, majority of which were depressive disorders, emotional lability, and anxiety disorders. More than half of the events occurred in 10-19 year-olds. There were 368 completed suicides, 78.8% of which were male (incidence of 5.6-8.4 per 100,000 patients). Interestingly, this rate is lower than that of the general population (14.5 per 100,000)8. A 2017 JAAD meta-analysis showed that depression actually decreased with isotretinoin treatment9.
#3 | FACT OR FICTION?
Patients who are able to become pregnant need an office or lab-based pregnancy test each month.
FICTION
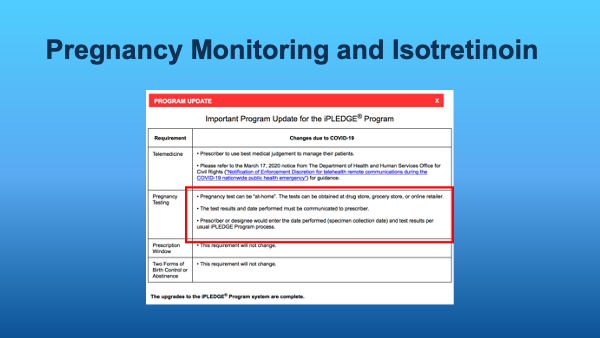
With the emergence of the COVID-19 pandemic, any patient required by iPLEDGE rules to take a monthly pregnancy test can now take one at home.
Why are pregnancy tests indicated on a monthly basis?
Isotretinoin causes malformations in the developing fetus, affecting the craniofacial, cardiovascular, thymic, and central nervous systems1. In the early years of isotretinoin use, these defects were reported in nearly 50% of full term pregnancies exposed to this medication in the first trimester (along with stillbirths, spontaneous abortions). At this time, there is NO designated minimal safe dose for use in pregnancy—thus it is category X.
The iPLEDGE system (established 2006) is a federal registry generated as a risk management system for patients on isotretinoin, specifically to mitigate the risk of fetal exposure to isotretinoin. All patients on isotretinoin, as well as prescribers, pharmacies, and wholesalers are a part of this registry. A 2019 study published in JAMA Dermatology reviewed FDA-reported pregnancies, abortions, and fetal defects amongst women on isotretinoin from 1997 to 201710. The annual incidence of pregnancies was between 0.33% and 0.65% with the peak occurring in 2006 (year iPLEDGE established). The incidence of pregnancies and pregnancy-related adverse events has since decreased, however, still persists with an average of 218-310 reported pregnancies per year.
When is a pregnancy test needed?
-
- iPLEDGE registration day
- 30 days after registration (then patients have 7 days to get their medication)
What kind of pregnancy tests are acceptable (hint: look at Dr. Lester’s slide below!)?
-
- Urine hCG at HOME (new update during the pandemic). This can cost patients $$$.
Remember that the practitioner is still ultimately responsible for the patient’s negative pregnancy test. Various offices handle “home pregnancy tests” differently: patients can send in photo vs telehealth visit, etc.
-
- Urine hCG IN OFFICE
- Serum hCG (lab)
Every time a patient is given an initial and subsequent prescription of isotretinoin, the provider must confirm patient counseling, as well as pregnancy test results and contraception methods used (if determined to be a person who is able to get pregnant by iPLEDGE rules). Prescriptions then must be filled within 7 days of the office visit/pregnancy test.
#4 | FACT OR FICTION?
There are disparities in access to isotretinoin.
FACT
A recent 2020 JAMA Dermatology Article reviewed the medical charts of 29,928 patients who were diagnosed with acne and followed for at least 1 year in the healthcare system11. Of this large group, 63.9% were female, 67.8% were Non-Hispanic White, 7.9% Black, 2.8% Asian, 5.5% Hispanic, and 15.7% other.
Results demonstrated that those with Medicaid, who were female or black, were less likely to receive a prescription for isotretinoin. Black patients are more likely to see a dermatologist for acne but get fewer systemic treatments. Men were almost 2.5X more likely than Female patients to receive prescriptions for isotretinoin. Patients with Medicaid (compared to commercial insurance) were less likely to see a dermatologist at all! On top of that, they were less than half as likely to be prescribed topical retinoids, oral antibiotics, spironolactone, and isotretinoin.
We need to be aware of these disparities when considering isotretinoin for our patients. Even more importantly, those with darker skin types are readily affected by post-inflammatory hyperpigmentation as a result of their acne, which can be devastating, long-lasting, and recalcitrant to treatment. This begs a case to consider aggressively treating acne in our patients with darker skin tones to minimize these unwanted sequelae of chronic, inflammatory, and undertreated acne.
Don’t be afraid to bust through barriers to care!
#5 | FACT OR FICTION?
Special considerations are needed when treating a trans person with Isotretinoin
FACT
Let’s break this down. As a baseline, transgender individuals have an increased risk of depression, suicidal ideation, and self-harm compared to their cisgender counterparts. This, compounded with mental health concerns associated with acne should be considered in transgender patients12. Regarding iPLEDGE, patients must register with their sex assigned at birth, eg. Female or Male. These options may compromise an individual’s right to self-identify. Proposed restructuring of the categorization includes “reproductive ability” without a specific gender identification13. Given that, any person registered as a female must take monthly pregnancy tests, even if on testosterone therapy. Further, any person registered as a female must use two forms of birth control or agree to abstinence.
A provider should become a “champion of bedside manner” and have an open conversation with the patient regarding14:
-
- Reproductive status
- Sexual orientation
- Anatomical considerations
Practitioners should also use the preferred name and personal pronoun of the patient as well as acknowledging more sensitive parts of the physical exam.
#6 | FACT OR FICTION?
Isotretinoin exposure significantly increases risk of inflammatory bowel disease (IBD)
FICTION
The risk of IBD in patients on isotretinoin is overall low, but slightly higher than controls. One study reported the incidence of IBD in patients with exposure to isotretinoin in the past 6 months to be about 2.5 MORE cases per 10,000 compared to controls 15. When following patients for at least 1 year after their initial isotretinoin prescription, the difference in incident cases of IBD between isotretinoin-exposed patients and controls became non-significant. Two theories for this potential association are as follows:
Theory 1: Surveillance bias— since providers are seeing their isotretinoin patients monthly— does this give more opportunity to screen for IBD, thus potentially leading to an increased rate of diagnosis/rising reported incidence?
Theory 2: Tetracyclines may contribute to the development of IBD. Prior doxycycline use may be an important confounder when considering incidence of IBD in isotretinoin-exposed patients. When controlling for tetracycline exposure, researchers found a partial reduction in the association between isotretinoin and IBD.
Other important considerations for isotretinoin
When is surgery safe on or after isotretinoin therapy?
There is controversy whether isotretinoin impairs wound healing— there may be increased risk for hypertrophic scarring as well as post-inflammatory hyper- or hypopigmentation. Waxing can cause increased epidermal stripping. A recent article reviewed published guidelines on these indications16. There was a particular focus on those from the American Society for Dermatologic Surgery (ASDS), American Academy of Dermatology (AAD), European Medicines Agency (EMA)—the equivalent of the FDA for Europe, as well as French and Canadian guidelines.
Here’s what they found:
-
- The ASDS (2017) says there is insufficient evidence to delay non-ablative lasers and superficial peels. Focal/superficial manual dermabrasion may be safe if performed by an experienced clinician. Avoid full face dermabrasion or mechanical dermabrasion with rotary devices for 6 months after isotretinoin discontinuation.
- The AAD (2016) recommends avoiding elective procedures for 6-12 months after the completion of isotretinoin, however, these guidelines should be considered on a case-by-case basis.
- The EMA recommends: avoiding dermabrasion, waxing, and cutaneous lasers for 6 months after isotretinoin use; although they state that these guidelines are open for future review/revision.
Wow! What high yield factoids we learned. Dr. Lester basically gave us the 411 on all important considerations we should think about when prescribing isotretinoin for acne. Here are Dr. Lester’s “take-home points:”
-
- Most agree that labs should be checked at baseline and one month after max dose is reached
- Patients on isotretinoin are vulnerable to mental health concerns
- At-home pregnancy tests are acceptable during the pandemic
- There are disparities in access to systemic acne treatment
- There are special considerations to be made when treating trans people with isotretinoin
- There appears to be a slight increase in risk of IBD but clinical significance is debated
How often do you check labs during isotretinoin therapy?
Have any pearls on isotretinoin that you would like to share?
Please share your comments at the end of this post, and on Instagram @nextstepsinderm!
References
-
- Wolverton SE, Wu JJ. Comprehensive dermatologic drug therapy. Fourth. ed. Philadelphia: Elsevier; 2020.
- Banks PA, Freeman ML, Practice Parameters Committee of the American College of G. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379-2400.
- Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48(3):195-203.
- Vallerand IA, Lewinson RT, Farris MS, et al. Efficacy and adverse events of oral isotretinoin for acne: a systematic review. Br J Dermatol. 2018;178(1):76-85.
- Hansen TJ, Lucking S, Miller JJ, Kirby JS, Thiboutot DM, Zaenglein AL. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75(2):323-328.
- Sundstrom A, Alfredsson L, Sjolin-Forsberg G, Gerden B, Bergman U, Jokinen J. Association of suicide attempts with acne and treatment with isotretinoin: retrospective Swedish cohort study. BMJ. 2010;341:c5812.
- Singer S, Tkachenko E, Sharma P, Barbieri JS, Mostaghimi A. Psychiatric Adverse Events in Patients Taking Isotretinoin as Reported in a Food and Drug Administration Database From 1997 to 2017. JAMA Dermatol. 2019.
- Suicide and Self Inflicted Injury. CDC/National Center for Health Statistics.Published 2020. Updated 4/20/2020. Accessed 8/14/2020, 2020.
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: A systematic review and meta-analysis. J Am Acad Dermatol. 2017;76(6):1068-1076 e1069.
- Tkachenko E, Singer S, Sharma P, Barbieri J, Mostaghimi A. US Food and Drug Administration Reports of Pregnancy and Pregnancy-Related Adverse Events Associated With Isotretinoin. JAMA Dermatol. 2019.
- Barbieri JS, Shin DB, Wang S, Margolis DJ, Takeshita J. Association of Race/Ethnicity and Sex With Differences in Health Care Use and Treatment for Acne. JAMA Dermatol. 2020.
- Campos-Munoz L, Lopez-De Lara D, Conde-Taboada A, Fueyo Casado A, Lopez-Bran E. Depression in transgender adolescents under treatment with isotretinoin. Clin Exp Dermatol. 2020;45(5):615-616.
- Boos MD, Ginsberg BA, Peebles JK. Prescribing isotretinoin for transgender youth: A pledge for more inclusive care. Pediatr Dermatol. 2019;36(1):169-171.
- Richer V, Kuritzky LA. Considerations in Treating Severe Acne with Isotretinoin in Transgender Men. J Cutan Med Surg. 2020:1203475420930224.
- Wright S, Strunk A, Garg A. Risk of new onset inflammatory bowel disease among acne vulgaris patients exposed to isotretinoin. J Am Acad Dermatol. 2020.
- Dessinioti C, Zouboulis CC, Bettoli V, Rigopoulos D. Comparison of guidelines and consensus articles on the management of patients with acne with oral isotretinoin. J Eur Acad Dermatol Venereol. 2020.
This information was presented by Dr. Jenna Lester at the GW Virtual Appraisal of Advances in Acne Conference held July 30th, 2020.
Did you enjoy this article? Find more on Medical Dermatology here.

