When we think about skin, a few things come to mind: protection, temperature regulation, sensation. However, there is growing interest in the microbiome of the skin. Our skin flora can be likened to an invisible ecosystem. Similar to fingerprints, it is unique to each individual. The complexity of the skin microbiome is continuing to be researched. This research is paving the way to improve our understanding of the relationship between acne and dysbiosis.
At the GW Virtual Appraisal of Advances in Acne Conference, Dr. Adam Friedman discussed Microbiome Manipulation in the Management of Acne. His lecture provided insight on the microenvironment of the skin and how the diversity in skin flora can affect disease processes such as acne.
First, let me share a few pearls from Dr. Friedman’s lecture.

The skin is a physical barrier against invasion by pathogenic organisms and foreign substances. The skin is also an ecosystem, host to a variety of microorganisms that are typically harmless.

The habitat of the skin varies topographically and there are several factors that contribute to this unique variation among individuals.

The cutaneous immune system modulates and can be modulated by these commensal microorganisms. Dysbiosis, which directly refers to decreased microbial diversity, is directly linked to dysregulation of the skin immune response is evident in several skin disorders.
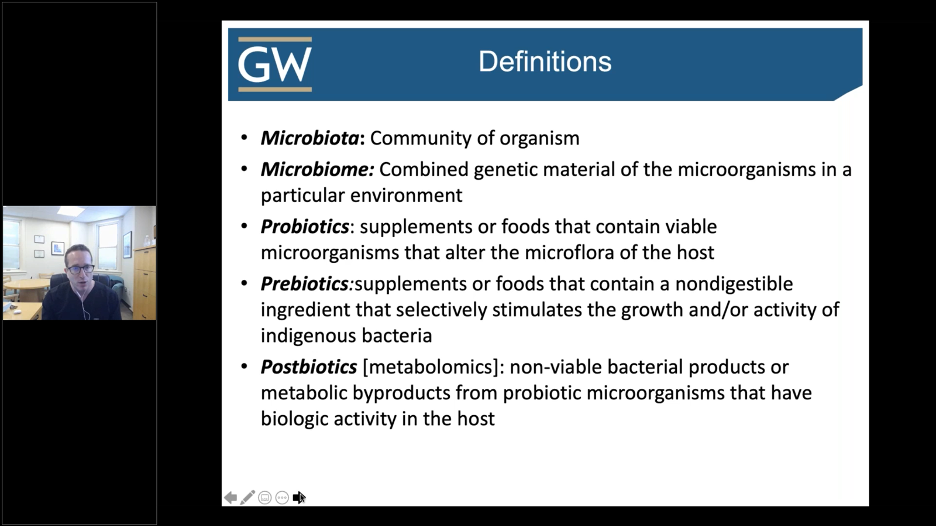
Before we can begin our discussion on the skin microflora and its impact on acne, we must define a few key terms. Below is a snapshot from Dr. Friedman’s lecture.
When we think about the skin barrier, we usually think about the hydrophilic corneocytes and hydrophobic lipids that make up this security guard for your skin. However, we do not necessarily think about the invisible barrier that protects the skin, which is composed of numerous microorganisms. Both of these components are vital to the structural stability of the skin barrier. If this invisible barrier is disrupted, this may lead to skin disease. Why are these commensal organisms so important?
Role of commensal organisms on the skin:
-
- Inhibit colonization of foreign pathogens
- Maintain the pH balance of the skin
- Inhibit inflammation
We all have a unique skin microbiome. What contributes to the variability of the skin microbiome?
Factors contributing to individual variations in skin microflora:
-
- Gender
- Age
- Environment
- Lifestyle
- Genetics
- Underlying medical conditions
However, there are other exogenous sources of dysbiosis, including:
-
- Steroids
- Antibiotics
- Topical retinoids
- Harsh soaps
- Chemical and physical exfoliants
- Resurfacing techniques
As dermatologists, we must be aware of how our interventions can contribute to dysbiosis as well.
Let’s transition to skin microflora and its impact on disease. A wide range of skin disorders are hypothesized to arise in part due to a microbial component.
The skin microbiome plays a role in several diseases:
-
- Atopic Dermatitis
- Psoriasis
- Rosacea
- Acne
We will focus our attention on acne. Acne is a disease of the pilosebaceous unit with multifactorial pathogenesis; sebum overproduction, abnormal keratinization, inflammation.
Cutibacterium acnes (formerly Propionibacterium acnes) is also implicated as a pathogenic factor in acne. C. acnes is a lipophilic, Gram-positive, anaerobic bacterium that colonizes the skin. It is known to prevent colonization of opportunistic pathogens such as MRSA and help maintain the acidic pH of the skin. So, when does a commensal organism become a problem?
Bacteria dominate the skin follicular microbiome, especially in patients with acne.
Researchers performed ultra-deep metagenomic sequencing of follicular microbiota in both acne patients and age-matched individuals without acne. They found five main bacterial phyla in the skin samples. Normal skin microflora in areas with abundant pilosebaceous follicles consists principally of Actinobacteria, Staphylococcus spp, and Malassezia spp.
C. acnes (an actinobacterium) was the most prevalent and abundant in both acne and non-acne patients. However, in acne patients, there is a disruption in the normal bacterial composition of the skin. Not only do we see an increase in the concentration of acnes, there is also an increase in Staphyloccocal species (Firmicute phyla). Below is a snapshot from Dr. Friedman’s lecture depicting these differences.
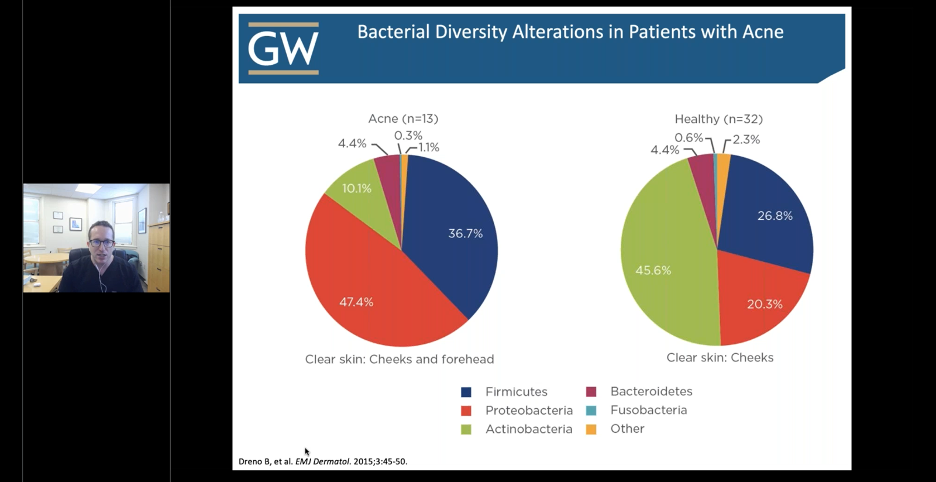
Although the relative abundance of C. acnes was similar in acne vs non-acne patients, there was found to be variability in strain population that were significantly different in the two cohorts.
There are different strains of C. acnes
There are at least 70 different strains of C. acnes, which can be protective or pathogenic. Certain strains were highly associated with acne, and other strains were enriched in healthy skin. Different C. acnes strains induce distinct immune responses. Protective strains secrete interleukin-10, an immune regulatory cytokine that inhibits proinflammatory cytokine function. Pathogenic strains are overexpressed in acne and secrete inflammatory cytokines such as interferon-gamma (IFN-γ). IFN-γ is a pro-inflammatory cytokine that induces macrophage activation, and stimulation of NK cells and neutrophils.
Pathogenic strains of C. acnes can change sebum composition as well. C. acnes transforms sebum into short-chain fatty acids (SCFA). The SCFAs produced by C. acnes inhibits enzymatic activity of histone deacetylases in keratinocytes, which normally function to keep toll-like receptors (TLR) in check. TLR are expressed on the surface of cells and function to recognize pathogen-associated molecular patterns to instigate an immune response. HDACs help regulate TLR function to prevent recognition of commensal organisms as foreign. The SCFAs cause uncoupling of HDACs from TLRs thereby causing an immune response against self-antigens, in this case, commensal skin microbiota. Thus, SCFA-induced cytokine expression by C. acnes may regulate immune tolerance of commensal organisms in the skin.
So, we’ve learned that C. acnes may induce a persistent inflammatory response in our acne patients. Could antibiotics serve as more than an anti-inflammatory agent in the management of acne?
Isotretinoin and tetracycline treatments modify the skin microbiota in acne.
There have been several studies examining the effect of systemic antibiotics and oral retinoids on skin flora. Although isotretinoin has no direct antimicrobial effects, it reduces sebum production, thereby altering the microenvironment, resulting in a reduced concentration of C. acnes. Tetracyclines are broad-spectrum antibiotics that are most commonly used to treat acne. This class of antibiotics are highly effective against various pathogens and reduce the number of C. acnes and staphylococci on acne patients’ skin.
Non-selective antibiotics can have deleterious effects on the normal microbiota.
Although the use of antibiotics in the treatment of acne is mainly for anti-inflammatory purposes, targeting C. acnes and staphylococci is a reasonable approach in therapy. However, tetracyclines are broad-spectrum antibiotics that disturb the other commensal flora of the skin. Novel narrow-spectrum antibiotics make it possible to treat acne without disturbing the normal microbiota.
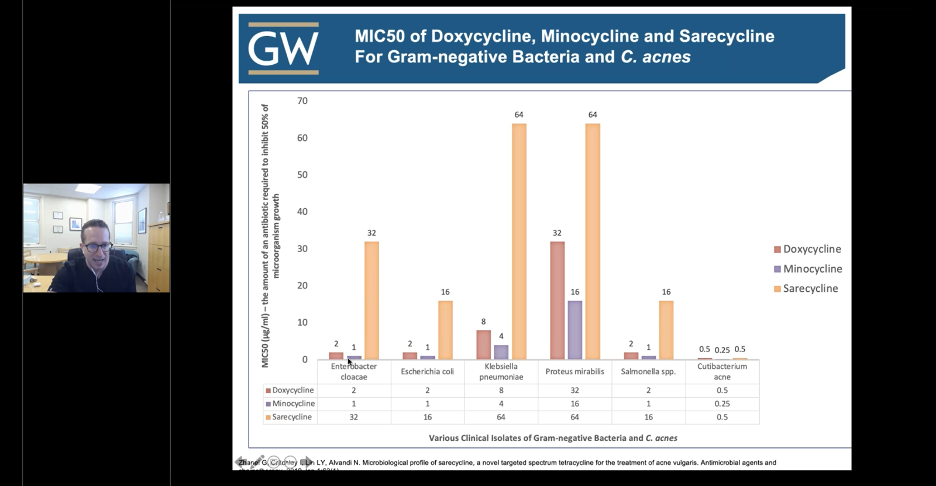
Sarecycline is the first narrow-spectrum tetracycline-class antibiotic developed for acne treatment. Sarecycline exhibits targeted antibacterial activity against C. acnes and is less active against aerobic Gram-negative bacilli associated with the normal human intestinal microbiome compared to doxycycline or minocycline. Not only is sarecycline as effective as doxycycline or minocycline in the treatment of acne, but the propensity of C. acnes to develop resistance to sarecycline is also low. Below is a snapshot from Dr. Friedman’s lecture illustrating the concept of narrow-spectrum when comparing different tetracycline antibiotics and selective targeting of microorganisms.
Prebiotics and the Skin
What about using prebiotic moisturizers in acne patients? In a study by Wang, Y, et al. (doi:10.1007/s00253-013-5394-8) it was shown that normal skin microbiota can mediate fermentation of glycerol to enhance inhibitory effects on P. acnes growth. Specifically, Staphylococcus epidermidis can ferment glycerol and produce succinic acid, a SCFA that can effectively inhibit the growth of P. acne.
As far as probiotics, Rahmayani T, et al. (doi:10.3889/oamjms.2019.718) found an increase in serum IL-10 levels in acne patients who took oral probiotics. Thus, combination use of both pre- and probiotics can be beneficial in acne patients.
In summary, nurturing the skin microflora as part of our skin barrier can aid patients in managing their acne.
This information was presented by Dr. Adam Friedman at the GW Virtual Appraisal of Advances in Acne Conference held July 30th, 2020.
Did you enjoy this article? Find more on Medical Derm Topics here.

